0820
Feasibility of Quantifying Myelin Water Fraction in the Fetal Guinea Pig Brain1Medical Biophysics, Western University, London, ON, Canada, 2Robarts Research Institute, Western University, London, ON, Canada, 3Physiology and Pharmacology, Western University, London, ON, Canada, 4Obstetrics and Gynaecology, Western University, London, ON, Canada, 5Maternal, Fetal & Newborn Health, Children's Health Research Institute, Lawson Research Institution, London, ON, Canada
Synopsis
Myelin water fraction (MWF), a validated marker for myelin lipid, can be used to assess fetal myelination in vivo. Thus, this study aims to demonstrate the feasibility of quantifying fetal MWF. 11 pregnant guinea pigs with 38 fetuses were imaged in a 3T MRI. mcDESPOT was used to quantify MWF in the corpus callosum (CC) of the maternal brain and the CC and fornix (FOR) of the fetal brain. MWF maps were successfully generated in all fetal brains, confirming its feasibility. Maternal MWF results and comparison of fetal CC and FOR MWF results are consistent with published results.
Introduction
Myelin, a lipid-rich substance that insulates nerve cells’ axons, is essential for healthy brain development as it facilitates long-range neuronal communications processes supporting higher-order cognitive, sensory, and motor functions1. In the human fetus, the earliest evidence of the myelin sheath is seen at approximately 18 weeks gestation, followed by an increasing rate of myelination throughout the brain that continues postnatally2-4. It is important to assess fetal myelin content to understand the impact of pathologies, such as intrauterine growth restriction (IUGR), on myelination5, but we have yet to quantify myelin content in vivo.Myelin water imaging (MWI) separates signals from myelin water and intra-/extra-cellular water, with signal amplitudes proportional to the relative amounts of water in each environment1. The primary measurement of MWI is myelin water fraction (MWF), the ratio of signal from protons in myelin water to total signal from all protons in water1. MWF has been validated as a strong marker for myelin lipid6, and multiple groups have used MWI in children and neonates to quantify their myelin content7,8.
In the present study, we sought to quantify myelin content in the fetal brain using guinea pig, especially given that the guinea pig, unlike other rodents, is classified as a prenatal brain developer9.
Methods
This study was performed with the approval of the institution’s Animal Care Committee. 11 pregnant chow-fed guinea pigs (~60 days gestation), with 38 fetuses, were anesthetized using isoflurane and imaged in a 3 T MRI (GE Discovery MR750). T2-weighted 1H images were obtained of the entire maternal guinea pig; these images were matched to the driven-equilibrium single-pulse observation of T1 and T2 (DESPOT1/2) volumes.Eight spoiled gradient echo (SPGR) volumes (TR: 4.6 – 5.9 ms, TE: 1.9 – 2.0 ms) for DESPOT1 were acquired at varying flip angles (FA: 2° – 16°, increasing increments of 2°) in approximately 7-12 minutes. Two sets of eight balanced steady-state free precession (bSSFP) volumes were acquired at various FA (FA: 8° – 64°, increasing increments of 8°), and at 0° and 180° phase increments; the 16 bSSFP volumes (TR: 6.4 – 7.4 ms, TE: 3.2 – 3.7 ms) for DESPOT2 were acquired in approximately 20-40 minutes; all DESPOT1/2 acquisitions had field-of-view: 23-24 cm, voxel size: 0.7 x 0.7 x 0.7 – 1 x 1 x 1 mm, acceleration: 2x. A mask of the maternal and fetal guinea pig brains was manually generated from the T2-weighted images using FSL10.
Quantitative Imaging Tools11 was used to generate the T1 map, T2 map, and MWF maps for each maternal and fetal guinea pig brain. Multicomponent driven-equilibrium single-pulse observation of T1/2 (mcDESPOT) was used to reconstruct each brain’s MWF map.
Using 3D Slicer12, regions of interest (ROIs) with a diameter of 1 mm were placed in the corpus callosum (CC) of each maternal brain. ROIs with a diameter of 0.5 mm were then placed in the CC and fornix (FOR) of each fetal guinea pig brain. The ROIs were placed using the T2-weighted images and transferred to the MWF maps to quantify the MWF values. The mean MWF value of the fetal CC and FOR were compared using a t-test (α=0.05).
Results
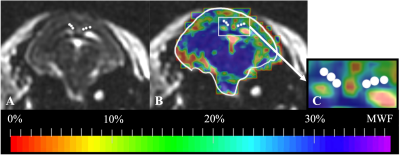
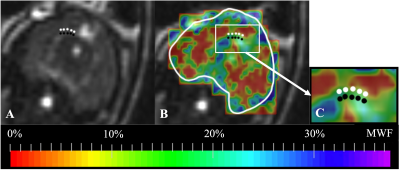
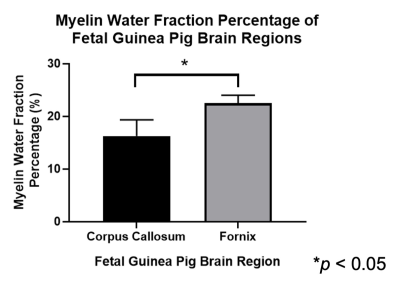
MWF maps were successfully generated for each maternal and fetal guinea pig brain. The mean MWF percentage in the maternal guinea pig brain CC is (mean ± standard deviation) 34.1 ± 2.40% (Figure 1). The mean MWF percentage in the CC and FOR of the fetal guinea pig brain is 16.3 ± 3.01% and 22.5 ± 1.5%, respectively. The mean MWF percentage of the fetal FOR was significantly greater than the fetal CC (p < 0.05) [Figures 2 & 3].Discussion
MWF maps were successfully generated in all fetal guinea pig brains, thus, confirming the feasibility of MWI in the fetal environment. We found the DESPOT1/2 scans with a 0.7 mm isotropic resolution to be ideal as these scans were acquired in approximately 40 minutes and the MWF reconstruction took approximately 3 hours; furthermore, MWF maps of a higher resolution were too noisy for analysis.The mean MWF value of the maternal corpus callosum, which was used as an internal control, was consistent with previously published MWF values of the corpus callosum in adult guinea pig brains13, validating our fetal MWF results.
The mean MWF was significantly higher in the fetal FOR than the CC. These results are consistent with a study conducted by Piorkowska et al., where the percent area staining for myelin in fetal guinea pig brains was higher in the FOR than the CC14.
Limitations of this study include potential residual motion between the anatomical images used for ROI placement and MWF maps, partial volume effects when analyzing the MWF in different fetal brain regions, and the lack of histology to quantitatively validate the fetal MWF results. Histological analysis for staining for myelin in the collected maternal and fetal brains is planned to validate the fetal MWF results.
Conclusion
We have successfully produced MWF maps in the fetal guinea pig brain, demonstrating the feasibility of MWI to assess fetal brain myelin content.Acknowledgements
The co-authors would like to thank Karen Nygard for their help with the planned histological validation.References
1. MacKay A, Whittall K, Adler J, et al. In vivo visualization of myelin water in brain by magnetic resonance. Mag Reson Med. 1994;31(6):673-677.
2. Jakovcevski I, Zecevic N. Sequence of oligodendrocyte development in the human fetal telencephalon. Glia. 2005;49(4):480-491.
3. Harding R, Bocking AD. Fetal growth and development: Cambridge: Cambridge University Press;2001.
4. Mayer C, Joseph KS. Fetal growth: a review of terms, concepts and issues relevant to obstetrics. Ultrasound Obstet Gynecol. 2013;41(2):136-145.
5. Chase HP, Welch NN, Dabiere CS, et al. Alterations in human brain biochemistry following intrauterine growth retardation. Pediatrics. 1972;50(3):403-411.
6. Laule C, Moore GRW. Myelin water imaging to detect demyelination and remyelination and its validation in pathology. Brain Pathol. 2018;28(5):750-764.
7. Deoni SC. Quantitative relaxometry of the brain. Top Magn Reson Imaging. 2010;21(2):101-113.
8. Deoni SC, Mercure E, Blasi A, et al. Mapping infant brain myelination with magnetic resonance imaging. J Neurosci. 2011;31(2):784-791.
9. Morrison JL, Botting KJ, Darby JRT, et al. Guinea pig models for translation of the developmental origins of health and disease hypothesis into the clinic. J Physiol. 2018;596(23):5535-5569.
10. Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23 Suppl 1:S208-S219.
11. Wood TC. QUIT: QUantitative Imaging Tools. J. Open Source Softw. 2018;3(26):656.
12. Fedorov A, Beichel R, Kalpathy-Cramer J, et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn Reson Imaging. 2012;30(9):1323-1341.
13. Gareau PJ, Rutt BK, Karlik SJ, et al. Magnetization transfer and multicomponent T2 relaxation measurements with histopathologic correlation in an experimental model of MS. J Magn Reson Imaging. 2000;11(6):586-595.
14. Piorkowska K. Placental insufficiency resulting in fetal growth restriction alters synaptic development and neuronal myelination in guinea pigs at term. London, ON, Canada: University of Western Ontario; 2012.
Figures