0818
Multiparametric Modeling of Fetal MRI to Compare Lobular and Whole-Placental Segmentations for Fetal Growth Restriction1Kings College London, London, United Kingdom, 2University College London, London, United Kingdom
Synopsis
Babies with fetal growth restriction fail to grow properly in the womb and become hypoxic. They must be delivered prematurely to prevent stillbirth. DECIDE model fitting can determine properties which give information about the placental function and fetal blood saturation. This can be used to identify fetal growth restriction and manage the pregnancy effectively.
The aim is to compare DECIDE model fitting results for different mask segmentations. The placenta is segmented into lobules and used as a mask itself. Statistically significant differences of the fetal and placental properties between appropriately developing and growth-restricted pregnancies are determined.
Introduction
For healthy development in the womb, fetuses require an adequate supply of oxygen and nutrients1. Fetal development is affected by placental insufficiency. Fetuses fail to grow properly and have dangerously low oxygen levels and increased risk of cerebral palsy, fetal distress in labour, stillbirth, and cardiometabolic disease in later life2,3,4. Measuring placental function is challenging, but crucial as the placenta plays an important role in the exchange of oxygen, nutrients and waste products5. Placental function is measured indirectly via fetal growth and Doppler ultrasound6 rather than investigating the presence of chronic hypoxia5,7.New methods in quantitative MRI model fitting8 are able to determine properties which give information about placental function such as perfusion and fetal blood saturation2,9. This has the potential to identify pregnancies affected by placental insufficiency, so they can be managed appropriately and improve outcome.
Methods
Written consent was provided by all subjects and data anonymised. Subject MRI data was acquired with a 1.5T Siemens Avanto under free breathing. The voxel resolution was 1.9x1.9x6mm. Data was acquired as a combination of 7 b-values and 9 echo-times.Lobular (up to 6 per placenta as shown in figure 1) and placental segmentation was performed for 6 cases of FGR and 6 controls (itk-Snap). The growth-restricted data was grouped into pregnancies with normal Doppler’s, uterine artery Pulsatility Index (PI) >95th centile, and uterine and umbilical artery PI >95th centile (n=2 per group).
DECIDE model fitting3 was applied to each MRI dataset in MATLAB to compute multi-parametric maps (figure 2,3). The parameters of interest were pseudo diffusivity (d*), apparent diffusion coefficient (ADC), fetal T2, fetal (f) and maternal (v) perfusion volume. Average values were found for whole-placenta ROIs and segmented lobular regions.
The average mean value and full width at half maximum (FWHM) with their standard deviation were calculated and plotted to allow comparison between the groups. The mean gives information about the average parameter value, whilst the FWHM can be used to define the range of the parameter. The two-way Student t-test computed statistically significant differences between appropriately developing and growth-restricted cases at 0.05 significance level. One-way ANOVA testing was applied to investigate if results of lobular segmentations differ from whole placenta ROIs.
The mean and FWHM of each subjects lobules were plotted in separate figures. Average values and standard deviations were superimposed and colour-coded to represent the datasets for the appropriately developing and growth-restricted babies.
Results
The whole placental ROI average T2 values differ between appropriately developing and severe growth-restricted cases (control group=520.2±275.5ms, severe FGR group=887.5±579.1ms, two-way t-test rejects nullhypothesis with test statistic=-2.1498 and p-value=0.0428). The most distinctive group exhibiting significant differences for fetal T2 values were cases with raised uterine and umbilical Doppler as shown in figure 4 (control=520.2±275.5ms, normal Doppler=652.3±435.7ms, raised umbilical Doppler=821.1±636.8ms, raised uterine and umbilical Doppler=887.5 ±579.1ms).Fetal or maternal blood perfusion volume and ADC values show no significant difference between FGR and appropriately developing cases.
We did not find any significant differences between the mean placenta tissue properties of the lobular segmentations and the whole placenta. This is visible in figure 5. The nullhypothesis was not rejected during ANOVA testing for all properties (e.g. feto-placental perfusion fraction: p-value=0.0513, critical value=4.09, p<c).
We found no significant differences between the FWHM of MRI parameter between the lobular segmentations and the whole placenta. The nullhypothesis was not rejected after applying ANOVA testing for all properties (e.g. fetal blood perfusion volume: p-value=0.15, critical value=4.09, p<c).
However, the graph in figure 6 shows a significantly larger range (FWHM) for fetal blood perfusion volume of severe early-onset FGR compared to appropriately developing cases (lobular fetal blood perfusion volume: severe FGR=0.15±0.1, control=0.17±0.1; whole placenta fetal blood perfusion volume: severe FGR=0.5±0.6, control=0.03±0.1).
Discussion and Conclusion
Parametric MRI properties showed no significant difference between the mean values of individual lobules and whole placenta ROIs. The FWHM of the distribution of values in the lobules displays no significant difference from the whole placenta. All results for fetal and placental properties of lobular segmentations agree with those of the whole placenta ROIs. This indicates no difference for using lobular instead of placental segmentations.There was a similar degree of variability across all cases for normal and growth-restricted fetuses (figures 4 and 5). This gives information about the homogeneity of the lobules within a placenta, suggesting no significant difference in the overall homogeneity for placentas of appropriately developing and growth-restricted fetuses. Due to the time and effort required for lobule segmentations, it may be better to use placenta masks for future model fitting work.
This study is limited by the low number of cases investigated and variability in the number of lobules segmented for each placenta. The inclusion of additional cases and close investigation of the model-fitting assumptions could show more differences between appropriately developing and growth restricted pregnancies. Moreover, it could possibly determine statistically significant differences between the quantitative MRI properties of individual lobules and whole placenta segmentations.
Nonetheless, some differences were identified between appropriately developing and growth-restricted pregnancies. The data shows a significant difference of fetal T2 values for fetuses with raised uterine and umbilical Doppler. This could support the management of pregnancies identified with severe early-onset fetal growth restriction.
Acknowledgements
This research was supported by the Wellcome Trust (210182/Z/18/Z, 101957/Z/13/Z, 203148/Z/16/Z) and the EPSRC (NS/A000027/1).References
1. Schneider H. Ontogenic changes in the nutritive function of the placenta. Placenta. 1996; 17(1):15–26.
2. Melbourne, A., Aughwane, R., Sokolska, M., Owen, D., Kendall, G., Flouri, D., Bainbridge, A., Atkinson, D., Deprest, J., Vercauteren, T., David, A., & Ourselin, S. (2018, September 21). Separating fetal and maternal placenta circulations using multiparametric MRI. PubMed. https://pubmed.ncbi.nlm.nih.gov/30239036/
3. J. Gardosi, V. Madurasinghe, M. Williams, A. Malik, A. Francis, Maternal and fetal risk factors for stillbirth: Population based study, BMJ (Online) 346 (2013).
4. D. J. Barker, C. Osmond, J. Golding, D. Kuh, M. E. Wadsworth, Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease, British Medical Journal 298 (1989) 564–567.
5. Aughwane, R., Mufti, N., Flouri, D., Maksym, K., Spencer, R., Sokolska, M., Kendall, G., Atkinson, D., Bainbridge, A., Deprest, J., Vercauteren, T., Ourselin, S., David, A. L., & Melbourne, A. (2020, June 30). Magnetic resonance imaging measurement of placental perfusion and oxygen saturation in early-onset fetal growth restriction. Obstetrics and Gynecology.
6. J. Tay, G. Masini, C. M. McEniery, D. A. Giussani, C. J. Shaw, I. B. Wilkinson, P. R. Bennett, C. C. Lees, Uterine and fetal placental Doppler indices are associated with maternal cardiovascular function, American Journal of Obstetrics and Gynecology 220 (2019) 96.e1–96.e8.
7. Perrone, S., Santacroce, A., de Bernardo, G., Alagna, M., Carbone, S., Paternò, I., & Buonocore, G. (2019). Magnetic Resonance Imaging in Pregnancy with Intrauterine Growth Restriction: A Pilot Study. Disease Markers, 2019, 1-6.
8. Aughwane, R., Ingram, E., Johnstone, E., Salomon, L., David, A., & Melbourne, A. (2019, July 15). Placental MRI and its application to fetal intervention. Obstetrics and Gynecology. https://obgyn.onlinelibrary.wiley.com/doi/full/10.1002/pd.5526
9. Zeidan, A., Gilliland, P., Patel, A., Ou, Z., Flouri, D., Aughwane, R., Ourselin, S., David, A., & Melbourne, A. (2021). Quantitative Imaging of the Baby in Fetal Growth Restriction.
Figures
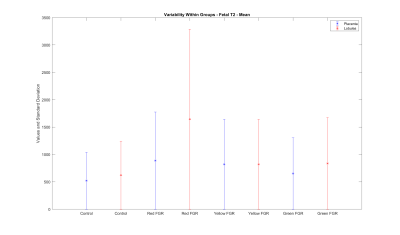
Average T2 values for cases with severe fetal growth restriction exhibit a clear distinction from the other cases.
(Control: appropriately developing fetuses, Red FGR: severe fetal growth restriction with uterine and umbilical Doppler, Yellow FGR: fetal growth restriction with umbilical Doppler, Green FGR: fetal growth restriction with normal Doppler)
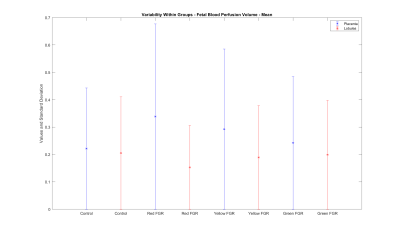
Mean values of fetal blood perfusion volume exhibit no difference between results for lobular segmentations and the whole placenta.
(Control: appropriately developing fetuses, Red FGR: severe fetal growth restriction with uterine and umbilical Doppler, Yellow FGR: fetal growth restriction with umbilical Doppler, Green FGR: fetal growth restriction with normal Doppler)
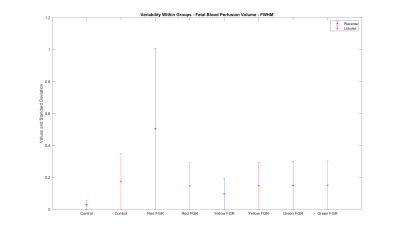
FWHM of fetal blood perfusion volume for lobular segmentations and the whole placenta exhibits a larger range for severely growth-restricted cases compared to normal cases.
(Control: appropriately developing fetuses, Red FGR: severe fetal growth restriction with uterine and umbilical Doppler, Yellow FGR: fetal growth restriction with umbilical Doppler, Green FGR: fetal growth restriction with normal Doppler)