0816
Effects of Life-Long Maternal Western Diet on Guinea Pig Placental Metabolism Across Gestation using [1-13C]pyruvate MRI1Medical Biophysics, Western University, London, ON, Canada, 2Radiation Oncology, University of California Los Angeles Health, Los Angeles, CA, United States, 3Physiology and Pharmacology, Western University, London, ON, Canada, 4Obstetrics and Gynaecology, Western University, London, ON, Canada, 5Division of Maternal, Fetal and Newborn Health, Children's Health Research Institute, London, ON, Canada
Synopsis
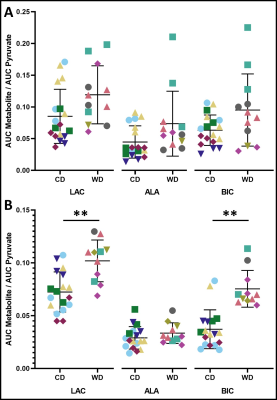
Effects of a life-long maternal Western Diet (WD: high fat and high sugar diet) on placental metabolism in 33- and 60-day gestation guinea pigs were investigated. Pregnant WD or control diet (CD) fed sows underwent T1-weighted and 13C hyperpolarized metabolic MRI at 3T. Metabolite mean signal intensities were measured in the placental volumes as a function of time and area under the curve (AUC) ratios were calculated for pyruvate and its metabolites: lactate, bicarbonate & alanine. The AUC ratios for lactate and bicarbonate were significantly higher in WD than CD groups, suggesting placental metabolic dysfunction.
Introduction:
Imbalanced diets have been enabled by the manufacturing of ultra-processed, high-energy foods 1. The Western Diet (WD) is an example of an imbalanced diet, high in refined sugar and saturated fats 2. Maternal consumption of this type of diet can negatively affect the fetal environment during a critical time of development, altering the formation of tissues and organs, which can have long-lasting effects on an individual. Consumption of diets high in fat and sugar during pregnancy is associated with metabolic changes in utero that underly metabolic abnormalities like elevated insulin secretion, adiposity, and NAFLD in post-natal life 3–8. Placental metabolism changes throughout pregnancy to meet the needs of the developing fetus 9,10. Therefore, observing metabolism throughout fetal development will increase understanding of the physiology in a healthy state and the metabolic changes in a compromised state such as one exposed to a WD.Hyperpolarized (HP) 13C-pyruvate MRI is an emerging metabolic imaging method that substantially increases the MRI signal for labelled substrates that intersect key reactions, including glycolysis, the tricarboxylic acid (TCA) cycle, and amino acid biosynthesis 11. Typically, pyruvate is preferentially metabolized through aerobic metabolism as it yields more energy than anaerobic metabolism; however, under stress, the cell may utilize most of the pyruvate for the generation of lactate even in the presence of sufficient oxygen 12. This effect is a hallmark of cellular metabolic dysfunction, called aerobic glycolysis, and occurs in proliferating tissues, such as tumours. We hypothesize that aerobic glycolysis may be observed in placentae and will manifest as an increase in lactate production in WD exposed placentae relative to Control Diet (CD) exposed placentae. This project observes how maternal WD consumption affects placental [1-13C]pyruvate metabolism from mid- to late-gestation.
Methods:
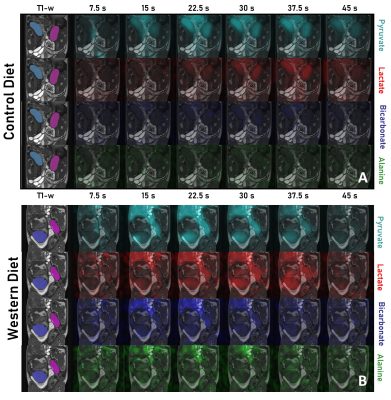
With approval from the institution's Animal Care Committee, twenty in-house-bred Dunkin-Hartley sows were weaned onto either a WD (33% carbohydrates, 46% fat, 21% protein, n=10) or a CD (60% carbohydrates, 18% fat, 22% protein, n=10). The pregnant sows were randomly allocated to imaging at two different time points: mid-gestation (33 ± 0.6 days, WD: n=5, CD: n=5) or late-gestation (60 ± 0.8 days, WD: n=5, CD: n=5). A total of 12 WD and 18 CD fetuses at both timepoints (litter size: 1-3 and 3-5, respectively) were imaged. One WD fetoplacental unit (60 days gestation) was not included in the study due to an anatomically abnormal placenta.Each sow underwent HP 13C MRI to acquire images of [1-13C]pyruvate and its metabolites using previously described methods 13,14. A modified IDEAL reconstruction was used to generate metabolic images of pyruvate and its metabolites: lactate, alanine and bicarbonate 13,15. The reconstructed metabolite signal intensities were corrected for the variation in the flip angle during HP image acquisition 13.
T1-weighted anatomical images were manually segmented in 3D Slicer to obtain volumes for each fetus and placenta. The mean signal intensities of [1-13C]pyruvate and its metabolites were measured in the placental volumes as a function of time (Fig. 1). The metabolic conversion rates from pyruvate to each of lactate (LAC), alanine (ALA) and bicarbonate (BIC) were estimated using the area under the curve (AUC) method 16. The fetal and placental data were compared between diets using a linear mixed model (LMM) controlled by the individual sow and including gestational age as a covariate.
Results:
At mid-gestation, there were no significant differences between the WD and CD groups for fetal and placental volumes (Fig. 2). The AUC ratios for LAC, ALA and BIC were not significantly different (p>0.05) between WD and CD groups (Fig. 3A).At late-gestation, there was no significant difference between the WD and CD groups in fetal volumes (Fig. 2A); however, the WD placental volumes were significantly higher (p<0.02) than the CD (Fig. 2B). The AUC ratios for LAC and BIC were significantly higher (p<0.01 and p<0.002, respectively) in the WD group compared to the CD group (Fig. 3B). The AUC ratio for ALA was not significantly (p>0.05) different between diets.
Discussion:
The AUC ratios are proportional to the metabolic conversion rate, and an indirect measurement of the enzyme concentration 17,18. The enzymes lactate dehydrogenase (LDH) and alanine aminotransferase (ALT) are responsible for catalyzing the reaction between pyruvate and lactate, and pyruvate and alanine, respectively. Pyruvate dehydrogenase (PDH) catalyzes the conversion from pyruvate to acetyl-CoA. The increased AUC ratios for LAC and BIC at late-gestation suggests increased enzyme activity for LDH and PDH, indicating altered pyruvate metabolism in WD-exposed placentae. There were no significant metabolic differences between diet groups at mid-gestation, suggesting that the metabolic effects of a poor maternal diet may not be observable at mid-gestation.This study was cross-sectional, limiting observation of each fetus and placenta to a single timepoint, instead of multiple timepoints throughout gestation.
Conclusion:
This study of guinea pigs investigated how fetal exposure to life-long maternal WD consumption affects placental pyruvate metabolism and fetal volume at two times during pregnancy. We conclude that the WD was associated with increased lactate and bicarbonate production in late gestation. We observed indications of dysfunctional metabolism in WD-exposed placentae, aligning with our hypothesis that altered pyruvate metabolism may exist in proliferating tissues such as the placenta.Acknowledgements
This work was supported by: Children’s Health Research Institute (CHRI) Trainee Award, funded by the Children’s Health Foundation, the Canadian Institutes of Health Research (grant number 162308) and the National Institutes of Health (grant number 1U01HD087181-01). The authors acknowledge Hye Won Yun for her assistance with the segmentation of the images.References
1. Nardocci M et al. Consumption of ultra-processed foods and obesity in Canada. Can J Public Heal. 2019;110(1):4-14.
2. Cordain L et al. Origins and evolution of the Western diet: health implications for the 21st century. Am J Clin Nutr. 2005;81:341-54.
3. Borengasser SJ, et al. High fat diet and in utero exposure to maternal obesity disrupts circadian rhythm and leads to metabolic programming of liver in rat offspring. PLoS One. 2014;9(1):e84209.
4. Moon CM, et al. Metabolic biomarkers for non-alcoholic fatty liver disease induced by high-fat diet: In vivo magnetic resonance spectroscopy of hyperpolarized [1-13C] pyruvate. Biochem Biophys Res Commun. 2017;482(1):112-119.
5. Musial B, et al. A Western-style obesogenic diet alters maternal metabolic physiology with consequences for fetal nutrient acquisition in mice. J Physiol. 2017;595(14):4875-4892.
6. Samuelsson AM, et al. Diet-Induced Obesity in Female Mice Leads to Offspring Hyperphagia, Adiposity, Hypertension, and Insulin Resistance. Hypertension 2008;51:383-392.
7. Fernandez-Twinn DS, et al. Downregulation of IRS-1 in adipose tissue of offspring of obese mice is programmed cell-autonomously through post-transcriptional mechanisms. Mol Metab. 2014;3(3):325-333.
8. Tveden-Nyborg P, et al. Diet-induced dyslipidemia leads to nonalcoholic fatty liver disease and oxidative stress in guinea pigs. Transl Res. 2016;168:146–160.
9. Lain KY & Catalano PM. Metabolic changes in pregnancy. Clin Obstet Gynecol. 2007;50(4):938-948.
10. Sferruzzi-Perri AN, Higgins JS, Vaughan OR, Murray AJ & Fowden AL. Placental mitochondria adapt developmentally and in response to hypoxia to support fetal growth. Proc Natl Acad Sci U.S.A. 2019;116(5):1621-1626.
11. Ardenkjaer-Larsen JH, et al. Increase in signal-to-noise ratio of >10,000 times in liquid-state NMR. Proc Natl Acad Sci. 2003;100(18):10158-10163.
12. Heiden MGV, Cantley LC. & Thompson CB. Understanding the warburg effect: The metabolic requirements of cell proliferation. Science. 2009;324(5930):1029-1033.
13. Smith LM, Wade TP, Friesen-Waldner LJ & McKenzie CA. Optimizing SNR for multi-metabolite hyperpolarized carbon-13 MRI using a hybrid flip-angle scheme. Magn Reson Med. 2020;84(3):1510–1517.
14. Friesen-Waldner LJ, et al. Hyperpolarized [1- 13 C]pyruvate MRI for noninvasive examination of placental metabolism and nutrient transport: A feasibility study in pregnant guinea pigs. J Magn Reson Imaging. 2016;43(3):750-755.
15. Wiens CN, Friesen-Waldner LJ, Wade TP, Sinclair KJ & McKenzie CA. Chemical shift encoded imaging of hyperpolarized 13 C pyruvate. Magn Reson Med. 2015;74(6):1682-1689.
16. Larson PEZ, et al. Investigation of analysis methods for hyperpolarized 13C-pyruvate metabolic MRI in prostate cancer patients. NMR Biomed. 2018;31(11):e3997.
17. Daniels CJ, et al. A comparison of quantitative methods for clinical imaging with hyperpolarized (13)C-pyruvate. NMR Biomed. 2016;29(4):387-99.
18. Hill DK, et al. Model Free Approach to Kinetic Analysis of Real-Time Hyperpolarized 13C Magnetic Resonance Spectroscopy Data. PLoS One. 2013;8(9):e71996.
Figures
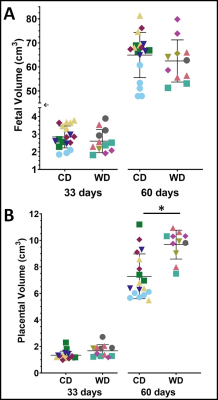
Fetal (A) and placental (B) volumes for control diet (CD) and Western diet (WD) groups at 33 days gestation and 60 days gestation. The WD placental volumes were significantly (p=0.013) larger than the CD at 60 days gestation. There is a break and scale change (←) in the (A) fetal volume axis to observe both timepoints. Data points with the same colour and shape within each group are fetuses from the same sow—one star (*) indicates a significant difference in volume (p<0.05).