0798
Towards 2D phase-contrast of tricuspid valve flow1Yale University, New Haven, CT, United States, 2National Institutes of Health, Bethesda, MD, United States, 3University of Oxford, Oxford, United Kingdom, 4Lund University, Lund, Sweden
Synopsis
Tricuspid valve flow evaluation is important for evaluation of regurgitation, used as a surrogate of pressures in the RV and as a criterion for evaluation of diastolic dysfunction. While 4D flow methods have interesting possibilities for retrospective valve flow evaluation its practical use is still limited. Here we present a method for direct tricuspid valve flow evaluation in a single breath-hold using 2D valve-following phase contrast sequence with a dynamic slice plane.
Background.
Valve diseases are an important cause of morbidity and mortality[1]. Specifically, tricuspid valve (TV) regurgitation can be detected in 80% of the general population and considered pathological (moderate or severe) in 15%[2]. Such TV leak is often due to elevated right ventricle (RV) pressure, commonly seen in pulmonary hypertension (PH)[3]. According to the current ACC/AHA guidelines, TV regurgitation is assessed with a comprehensive transthoracic echo (TTE) imaging with Doppler interrogation[1] of blood velocities. Moreover, recent studies suggest that cardiovascular MRI (cMRI) may be more accurate than TTE in assessing mitral valve (MV) regurgitation[4], [5]. Direct valve flow evaluation in cMRI is challenging and inadequate due to valvular displacement during the cardiac cycle; especially in the translating and rotating TV[6]. Therefore, cMRI cannot map TV regurgitant velocities. Recently, we developed 2D valve-following PC that follows the simple translational displacement of the MV throughout the heartbeat in a single breathhold;[7] it outperformed the standard static 2D phase contrast (2D PC), method for the measurement of blood velocities in accurately quantifying the MV flow. However, for use in TV flow and regurgitation evaluation, the method must be modified to follow the more complex TV translation and rotation, which is highly challenging. While 4D flow has been explored for quantitative TV and MV flow evaluation[8], [9], other limitations still confine its use to investigational studies. Thus, we present here a 2D PC sequence using 2 and 4 chamber RV cines to determine dynamic slice translation and rotation, for prospective valve-tracking PC (2DvtPC). We also present pilot data regarding TV flow and show its feasibility.Methods.
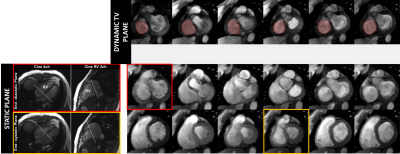
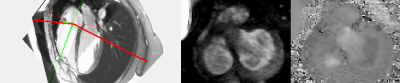
The protocol of acquisition of the proposed single breath-hold direct TV regurgitation evaluation by cMRI, using dynamic valve-plane PC is described figure 1. First, RV 2 and 4 chamber cines are acquired to serve as dynamic localizers for offline tracking of the TV using a previously developed semi-automatic feature tracking algorithm[10], [11]. The dynamic TV plane information required to modify the slice-prescription, defined by the TV center-point and its normal was determined by this tracking, and provided as input to the 2DvtPC sequence. During the acquisition, the sequence updates the slice geometry at each cardiac phase matching the slice center to the TV center, and the slice orientation to the TV orientation:$$ \overrightarrow{SL_t} = \overrightarrow{n_t}$$
$$\overrightarrow{PE_t} = \overrightarrow{RO_0} \times \overrightarrow{SL_t} $$
$$\overrightarrow{RO_t} = \overrightarrow{SL_t} \times \overrightarrow{PE_t} $$
with $$$\overrightarrow{n_t} $$$, $$$\overrightarrow{SL_t} $$$, $$$\overrightarrow{PE_t} $$$, $$$\overrightarrow{RO_t} $$$ the TV normal, the slice normal, the phase encode and the readout vectors, with $$$t$$$ indexing the cardiac frame and $$$\overrightarrow{RO_0}$$$ being the readout vector of the initial slice plane, planned by the user. cMRI was performed in 2 healthy subjects (3T, Siemens). PC data sets of the tricuspid valve were acquired during a single breath-hold with retrospective-gating using the following cMRI parameters: VENC=100cm/s, TR/TE/θ=5.31ms/3.2ms/15, matrix size 256x156, FOV 380mm, voxel size 1.48x1.48x6mm, 4 bipolar pairs per repetition and 42ms temporal resolution. This acquisition was performed for a static TV plane initialized in diastole, in systole and finally with a dynamic valve-tracking slice of the TV. Standard PC of the aorta and the main pulmonary artery were performed to compare resultant stroke volumes (SV) values. PC analysis was done using Segment software[12]. Motion correction was performed by pixel using the valve rotation and translation velocities[13].
Results.
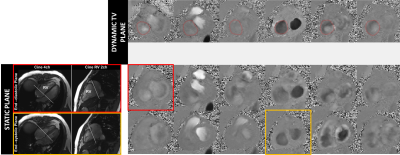
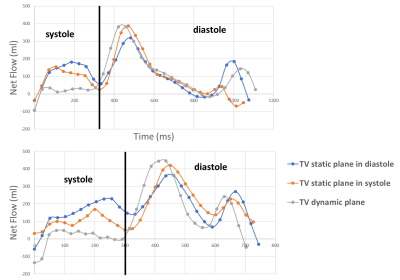
Resulting TV PC images of the magnitudes and phase for the static and dynamic acquisition are presented figure 2 and 3, at a few selected phases. Note that the images from the valve-tracking PC method matches (at end-diastole) the static PC images planned at end-diastole and matches at end-systole the static PC images planned at end systole. Figure 4 shows a tricuspid flow curve, presenting the flow by PC for the two static planes, and the valve-tracking PC. Note that there is slight regurgitation, observed in begin-systole, for both the static PC planned at begin-systole, and the valve-tracking PC. However, the valve-tracking plane yields a more physiological curve in general, with mainly no flow in systole, when the valve is shut. Note that the diastolic E and A waves, show characteristics similar to echocardiography velocity curves, and somewhat different than mitral flow patterns (e.g. the gradual tapering of the E-wave). In this small study net pulmonary artery and aortic flow agreed well (with bias +/-SD of -0.5 +/- 4.9mls), and the valve tracking TV flow agreed with PA flow (-3.5 +/- 13.4mls), and better than the static TV flow measurements (29.5mls +/-10.6 mls, and 19mls +/-14.1mls for static planes planned in begin- and end-systole).Conclusion.
Tricuspid flow is important for evaluation of valve regurgitation, used as a surrogate of pressures in the RV to assess of pulmonary hypertension, and as a criterion for evaluation of diastolic dysfunction. While 4D flow methods have interesting possibilities for retrospective valve flow evaluation its practical use is still limited. Here we present method for direct tricuspid flow evaluation in a single breath-hold. Its practical utility is even stronger, in light of recent methods for automated deep-learning analysis to track the TV plane[14] and could allow a full inline protocol for TV flow direct evaluation by 2d phase contrast.Acknowledgements
The authors acknowledge the support from National Heart Lung and Blood Institute, R01 HL155992.References
[1] C. M. Otto et al., “2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines,” Circulation, vol. 143, no. 5, Feb. 2021, doi: 10.1161/CIR.0000000000000923.
[2] J. P. Singh et al., “Prevalence and clinical determinants of mitral, tricuspid, and aortic regurgitation (the Framingham Heart Study),” Am. J. Cardiol., vol. 83, no. 6, pp. 897–902, Mar. 1999, doi: 10.1016/S0002-9149(98)01064-9.
[3] D. Mutlak, D. Aronson, J. Lessick, S. A. Reisner, S. Dabbah, and Y. Agmon, “Functional tricuspid regurgitation in patients with pulmonary hypertension: is pulmonary artery pressure the only determinant of regurgitation severity?,” Chest, vol. 135, no. 1, pp. 115–121, Jan. 2009, doi: 10.1378/chest.08-0277.
[4] S. Uretsky et al., “Discordance Between Echocardiography and MRI in the Assessment of Mitral Regurgitation Severity: A Prospective Multicenter Trial,” J. Am. Coll. Cardiol., vol. 65, no. 11, pp. 1078–1088, Mar. 2015, doi: 10.1016/j.jacc.2014.12.047.
[5] S. Uretsky et al., “A Comparative Assessment of Echocardiographic Parameters for Determining Primary Mitral Regurgitation Severity Using Magnetic Resonance Imaging as a Reference Standard,” J. Am. Soc. Echocardiogr., vol. 31, no. 9, pp. 992–999, Sep. 2018, doi: 10.1016/j.echo.2018.04.006.
[6] T.-T. Ton-Nu et al., “Geometric Determinants of Functional Tricuspid Regurgitation,” Circulation, vol. 114, no. 2, pp. 143–149, Jul. 2006, doi: 10.1161/CIRCULATIONAHA.106.611889.
[7] F. Seemann et al., “Valvular imaging in the era of feature-tracking: A slice-following cardiac MR sequence to measure mitral flow,” J. Magn. Reson. Imaging, vol. n/a, no. n/a, doi: 10.1002/jmri.26971.
[8] V. P. Kamphuis et al., “Automated Cardiac Valve Tracking for Flow Quantification with Four-dimensional Flow MRI,” Radiology, vol. 290, no. 1, pp. 70–78, Oct. 2018, doi: 10.1148/radiol.2018180807.
[9] V. P. Kamphuis et al., “In-scan and scan–rescan assessment of LV in- and outflow volumes by 4D flow MRI versus 2D planimetry,” J. Magn. Reson. Imaging, vol. 47, no. 2, pp. 511–522, 2018, doi: 10.1002/jmri.25792.
[10] M. Evin et al., “Left atrium wall tracking from MR images for strain assessment,” Comput. Methods Biomech. Biomed. Engin., vol. 17, no. sup1, pp. 14–15, août 2014, doi: 10.1080/10255842.2014.931055.
[11] J. Lamy et al., “Scan-rescan reproducibility of ventricular and atrial MRI feature tracking strain,” Comput. Biol. Med., vol. 92, pp. 197–203, Nov. 2017, doi: 10.1016/j.compbiomed.2017.11.015.
[12] E. Heiberg, J. Sjögren, M. Ugander, M. Carlsson, H. Engblom, and H. Arheden, “Design and validation of Segment--freely available software for cardiovascular image analysis,” BMC Med. Imaging, vol. 10, p. 1, Jan. 2010, doi: 10.1186/1471-2342-10-1.
[13] H. W. M. Kayser, B. C. Stoel, E. E. V. D. Wall, R. J. D. V. Geest, and A. D. Roos, “MR velocity mapping of tricuspid flow: Correction for through-plane motion,” J. Magn. Reson. Imaging, vol. 7, no. 4, pp. 669–673, 1997, doi: 10.1002/jmri.1880070410.
[14] R. A. Gonzales et al., “TVnet: Automated Time-Resolved Tracking of the Tricuspid Valve Plane in Long-Axis Cine Images with a Dual Stage Deep Learning Pipeline,” MICCAI, 2021.
Figures