0797
4D Flow MRI Analysis of Flow, Velocity, and Cardiac Flow Compartments in a Swine Model of Pulmonary Hypertension1Medical Physics, University of Wisconsin-Madison, Madison, WI, United States, 2Surgery, University of Wisconsin-Madison, Madison, WI, United States, 3Biomedical Engineering, University of Wisconsin-Madison, Madison, WI, United States, 4Medicine, University of Wisconsin-Madison, Madison, WI, United States, 5Radiology, University of Wisconsin-Madison, Madison, WI, United States, 6Edwards Lifesciences Foundation Cardiovascular Innovation and Research Center, University of California, Irvine, Irvine, CA, United States, 7Biomedical Engineering, University of California, Irvine, Irvine, CA, United States
Synopsis
Pulmonary hypertension is a known consequence of left heart failure. However, little is known about the pulmonary vascular and right ventricular changes caused by increased pulmonary venous pressure independent of left heart failure. By surgically banding the inferior pulmonary vein confluence in swine, we created pulmonary venous hypertension without damage to the left heart. Here, we report results from 4D flow MRI analysis of vessel flow, vessel velocity, cardiac flow compartments, and pressures measured via right heart catheterization in this novel model.
Introduction
Pulmonary hypertension (PH) is a common consequence of left heart failure and is caused by elevated left atrial pressure. PH is associated with increased mean pulmonary artery pressure (mPAP) as well as vascular changes that lead to increased pulmonary vascular resistance (PVR), both of which can be measured using right heart catheterization (RHC).1,2 As the disease progresses from isolated post-capillary to combined post- and pre-capillary PH (Cpc-PH), mortality rate rises due to right heart failure. In this pilot study, we evaluate severe PH independent of left heart failure in a swine model using 4D Flow MRI, aimed at pulmonary vascular and right heart effects of Cpc-PH.Methods
Invasive pulmonary vein banding (PVB)3 was performed in four male white swine (9.780.9kg) using a non-occlusive banding of the inferior pulmonary venous (IPV) confluence. A sham operation without band placement was performed on two male swine of similar weight (10kg, 11.7kg). Each swine was scanned 12-16 weeks after banding using cine bSSFP for cardiac function, CE MRA for vascular anatomy, and radially undersampled 4D flow MRI4 (3.0 T MRI scanner [GE Healthcare Discovery MR750, Waukesha, WI], TR=6.3ms, TE=2.1ms, isotropic resolution=1.95mm3, flip=8o, total scan time=13min). Swine PVB02, PVB04, and Sham 2 were scanned additionally one to two more times between 8-16 weeks post-surgery to facilitate longitudinal analysis. Cut planes were placed to measure velocities and flow in the vessels of interest using Ensight (Ansys, Canonsburg PA) and processed using custom software in Matlab (Mathworks, Natick MA). Flow pathline visualizations were also created in Ensight. RHC was performed within hours of each MRI exam. Cardiac flow compartment analysis5,6 was performed by segmenting both ventricles in bSSFP cine images from 16 weeks post-surgery across all cardiac phases. After registering the ventricle segmentations with 4D Flow magnitude data using ITK-SNAP, in house scripts were used for flow pathline generation and classification into 1) direct flow – enters and leaves the ventricle in one cardiac cycle, 2) delayed ejection flow – starts within but leaves the ventricle during systole, 3) retained inflow – enters during diastole and remains in the ventricle during systole, and 4) residual volume – starts within and remains in the ventricle.Results
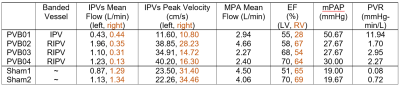
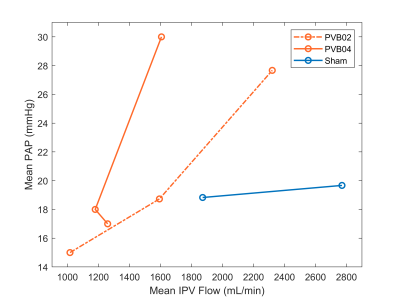
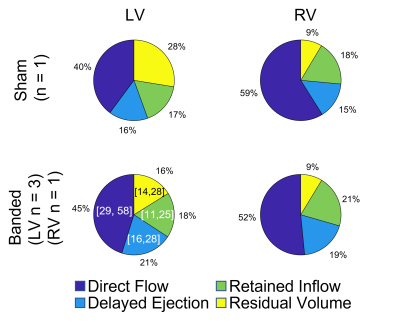
At week 16, swine body weights were: banded, 50.9±4.13 kg; shams, 58.7kg, 54.6kg. PVB01 was banded at the confluence of left and right inferior pulmonary veins (LIPV, RIPV), while the other 3 PVB swine had a band placed on the RIPV alone. Flow pathline visualization for PVB01, Sham 1, and PVB03 is shown in Figure 1, illustrating the effects of the band in each placement. All banded animals developed severe Cpc-PH without left heart involvement quantified by increased PVR but similar left ventricle ejection fraction (EF) compared with the shams. Flow, function, and pressure data are shown in Table 1. On average, banded swine suffered a 45% decrease in inferior pulmonary vein flow and 30% decrease in main pulmonary artery flow. Longitudinal comparison of mPAP and IPV mean flow is shown for swine scanned multiple times in Figure 2, indicating a rapid increase in banded swine mPAP with IPV flow between weeks 12 and 16 post surgery. Of the 5 swine with scans at 16 weeks post-surgery, 4 had sufficient image quality to analyze at least one of the ventricles using flow compartment analysis. All 4 were included in LV analysis, but 2 banded swine were excluded from RV analysis due to motion or unforeseen fiber optic catheter guide wire artifacts. Pie charts reporting compartment analysis are shown in Figure 3, illustrating an increase in LV (5%) and decrease in RV direct flow (7%) between sham and banded swine.Discussion
In this study, we used 4D Flow MRI to evaluate a novel swine model of severe PH independent of left heart failure. While the IPV confluence was targeted for banding, placement on the RIPV alone may provide less extreme PH cases for study. Swine with RIPV bands experienced lower mPAP pressure and were able to partially compensate for reduced RIPV flow through the LIPV compared with PVB01. The surgical banding procedure is challenging, involving working through a small (~5-6 cm) incision to locate and band the vessels: 4D Flow MRI allowed confirmation of success and location of the difficult procedure and quantification of blood flow and velocity. Longitudinal analysis shows that as the swine grows, mPAP rises to pathological levels due to increasingly severe impact of the banding. The banding model thus causes PH to develop over time, offering unique opportunity for insight into disease progression. While more swine are needed to validate the value of flow compartment analysis in this model, similar increased LV direct flow percentages have been reported in healthy swine during dobutamine stress tests.7 To our knowledge, this is the first use of RV flow compartment analysis in a swine model.Conclusion
In this study, we used 4D flow MRI to evaluate a swine model of induced Cpc-PH without left heart failure, enabling exclusive study of vascular and right heart changes with PH. Future work will increase the number of subjects, include histopathology, and analyze PH-specific hemodynamics in the main pulmonary artery using 4D flow visualizations.8Acknowledgements
We gratefully acknowledge GE Healthcare for research support of UW-Madison, and funding support from NIH R01HL147590. Dr. Oechtering receives funding from the German Research Foundation (OE 746/1-1).References
1. Assad TR, Hemnes AR, Larkin EK, et al. Clinical and Biological Insights Into Combined Post- and Pre-Capillary Pulmonary Hypertension. J Am Coll Cardiol. 2016;68(23):2525-2536. doi:10.1016/j.jacc.2016.09.942
2. Vonk Noordegraaf A, Groeneveldt JA, Bogaard HJ. Pulmonary hypertension. Eur Respir Rev. 2016;25(139):4-11. doi:10.1183/16000617.0096-2015
3. Pereda D, García-Alvarez A, Sánchez-Quintana D, et al. Swine model of chronic postcapillary pulmonary hypertension with right ventricular remodeling: Long-term characterization by cardiac catheterization, magnetic resonance, and pathology. J Cardiovasc Transl Res. 2014;7(5):494-506. doi:10.1007/s12265-014-9564-6
4. Johnson KM, Lum DP, Turski PA, Block WF, Mistretta CA, Wieben O. Improved 3D phase contrast MRI with off-resonance corrected dual echo VIPR. Magn Reson Med. 2008;60(6):1329-1336. doi:10.1002/mrm.21763
5. Corrado PA, Barton GP, Francois CJ, Wieben O, Goss KN. Sildenafil administration improves right ventricular function on 4D flow MRI in young adults born premature. Published online 2021. doi:10.1152/ajpheart.00824.2020
6. Eriksson J, Carlhäll CJ, Dyverfeldt P, Engvall J, Bolger AF, Ebbers T. Semi-automatic quantification of 4D left ventricular blood flow. J Cardiovasc Magn Reson 2010 121. 2010;12(1):1-10. doi:10.1186/1532-429X-12-9
7. Cesarovic N, Busch J, Lipiski M, et al. Left ventricular blood flow patterns at rest and under dobutamine stress in healthy pigs. NMR Biomed. 2019;32(1). doi:10.1002/NBM.4022
8. G R, U R, G K, H O, M F. Blood flow vortices along the main pulmonary artery measured with MR imaging for diagnosis of pulmonary hypertension. Radiology. 2015;275(1):71-79. doi:10.1148/RADIOL.14140849
Figures