0793
Reproducibility of 4D flow MR angiography in peripheral vessels: A combined phantom and in vivo study
Martin Sieben1, Andreas Deistung1, Maik Rothe1, Richard Brill1, Walter Alexander Wohlgemuth1, and Alexander Gussew1
1University clinic and policlinic for Radiology, University Hospital Halle (Saale), Halle (Saale), Germany
1University clinic and policlinic for Radiology, University Hospital Halle (Saale), Halle (Saale), Germany
Synopsis
Time-resolved 3D phase contrast MRA (4D flow MRA) is a technique providing morphological and quantitative 3D hemodynamic information of the blood vessel network without contrast agents and with a high temporal resolution. This study was performed to systematically validate flow measurements in peripheral vessels with high resolution, based on examinations in 4D flow phantom and in femoral vessels of healthy subjects. For this purpose, exams were run with varying spatial resolution and velocity encoding factors. It was shown, that the applied approach enables accurate and reproducible flow measurements by using venc factors of 120cm/s and isotrop spatial resolution of 1mm.
Introduction
Altering blood flow is characteristic for vascular diseases, such as aneurysms, thromboses or vascular malformations1. Therefore, assessment of hemodynamic properties, for example by using sonography or MR angiography (MRA), plays a central role for treatment, whose specification requires information on vessel type and flow dynamics2. However, despite its high temporal resolution, sonography becomes less accurate in deep vessels or different situations. Otherwise, contrast-enhanced MRA suffers from low temporal resolution and is not feasible for patients with an intolerance to contrast agents. Time-resolved 3D phase contrast MRA (4D flow MRA) is an alternative approach providing morphological and quantitative 3D hemodynamic information of the blood vessel network without contrast agents and with a high temporal resolution3,4. So far, 4D flow MRA has been mainly applied to characterize the blood flow within the heart and aortic vessels, with recent applications characterizing the vasculature of the head/neck region5. The application of 4D flow MRA in smaller peripheral vessels in the pelvis or legs or even in complex vascular malformations is challenging, as the required higher spatial resolution at large field-of-views is intrinsically associated with lower signal leading to lower accuracy. We conducted phantom and in vivo 4D flow MRA measurements to systematically investigate the influence of velocity encoding (venc) and spatial resolution on blood flow measurements in small peripheral vessels.Material and Methods
All measurements were performed on a 3T whole-body MR scanner (MAGNETOM Skyra, Siemens Healthcare, Erlangen, Germany) and by using a 4D flow MRA prototypical sequence (Siemens WIP 1106, CS 4D Flow Imaging), which allows ECG synchronized acquisition of dynamic phase contrast series with 3D flow encoding[6]. In phantom experiments, blood circulation was mimicked by using a flexible tube circuit (inner diameter: 8 mm), which will be flushed by a time varying, adjustable water flow (fig.1). The flow was generated by an external heart-lung machine pump (MOBYBOX, Hemovent, Aachen). Pressure and flow sensors were integrated into the flow circuit and used to synchronize flow measurements and derive flows and pressures as reference. Phantom data were collected with 4D flow MRA (TE: 2.73-3.22 ms; TR: 153-166ms, 9-10 flow cycle phases) using varying venc factors (venc: 60-200 cm/s) and varying isotropic voxels (voxel edge lengths: 0.8-1.6 mm). Phantom flow was adjusted between 30 and 85l/h (as measured with the flow sensors) and compared with corresponding 4D flow MRA values. In in vivo 4D flow experiments, blood flow was measured bilaterally in the femoral arteries and veins (fig.2) of eight male healthy subjects (23-42 y). Overall sequence parameters were similar to the phantom experiments, but using different venc factors (80, 120, 160 cm/s) and isotropic voxel sizes (0.89, 1.0, 1.33 mm). Using in-house software, the flow within the femoral vessels and the reproducibility in terms of variation coefficients were analysed. In phantoms, time step specific variation coefficients were calculated and then averaged for 50 equidistantly located analysis planes. In vivo, after computing the flow at five equidistantly located planes in each body side, the variation coefficient at flow maximum was calculated and then averaged among all planes and all subjects.Results and discussion
The relationship between measured and reference flow values in phantom measurements indicates appropriate accuracy of flow measurements with 4D flow MRA (fig.1). Due to inert flow sensor floats in the phantom, the measured flow values exhibited systematic but not significant aberrance from the reference values. The flow in the phantom was similar to the flow in the femoral vein (mean flow maximum: 24.3±9.1 l/h, diameter: 9.4±1.2 mm), which exhibits a low time variation and a homogenous velocity distribution within the vessel (compare fig.1 and fig.2). In contrast, arterial flow is characterized by a high intense bolus (approx. 200 ms relative to systole, mean flow maximum: 120±40 l/h, diameter: 10.6±0.7 mm), accompanied by turbulences, that become evident by regarding the heterogeneous inner-vessel velocity distribution (fig.2). The different venc factors had no significant effect on the measurement precision of flow in both the phantom and in the artery (fig.3). In contrast, the vein showed significantly elevated variation coefficients with increasing venc indicating growing influence of decreasing sensitivity due to decreased phase reliability at lower flows. In phantom, with decreasing spatial resolution partial volume effects increase progressively leading to decreasing reproducibility (fig.3). A similar behaviour was observed in the artery but not in the vein, where the higher influence of the noise becomes dominant for small voxel sizes.Conclusion
We performed phantom and in vivo experiments to investigate the effects of the velocity sensitivity and spatial resolution on the reproducibility of flow measurements in peripheral vessels with the 4D flow MRA technique. As indicated by variation coefficients lower than 6%, obtained with venc of 120 cm/s and voxel edge length of 1 mm, flow can be measured with sufficient reproducibility and appropriate accuracy. However, in order to achieve a better comparability between phantom and in vivo scans, further studies should be focussed on modifying the phantom to improve the simulation of temporal flow variations in arteries.Acknowledgements
We thank Siemens Healthcare for supporting this work, especially by providing the used prototypical 4D flow MRA sequence. We also thank the cardiology department of University Hospital Halle (Saale) for providing the external pump used in our flow phantom.References
- Zhang, C., et al., Blood flow and stem cells in vascular disease. Cardiovasc Res, 2013. 99(2): p. 251-9.
- Steiner, J.E. and B.A. Drolet, Classification of Vascular Anomalies: An Update. Semin Intervent Radiol, 2017. 34(3): p. 225-232.
- Markl, M., et al., Time-resolved 3D MR velocity mapping at 3T: improved navigator-gated assessment of vascular anatomy and blood flow. J Magn Reson Imaging, 2007. 25(4): p. 824-31.
- Markl, M., P.J. Kilner, and T. Ebbers, Comprehensive 4D velocity mapping of the heart and great vessels by cardiovascular magnetic resonance. J Cardiovasc Magn Reson, 2011. 13: p. 7.
- Schnell, S., C. Wu, and S.A. Ansari, Four-dimensional MRI flow examinations in cerebral and extracerebral vessels - ready for clinical routine? Curr Opin Neurol, 2016. 29(4): p. 419-28.
- Pathrose, A., et al., Highly accelerated aortic 4D flow MRI using compressed sensing: Performance at different acceleration factors in patients with aortic disease. Magn Reson Med, 2021. 85(4): p. 2174-2187.
Figures
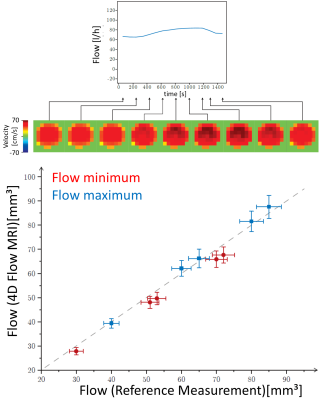
Time course of flow in the phantom with corresponding, time-resolved velocity distributions at a representative tube plane (top). Bottom: Comparison of reference and measured flow values, obtained at minima (red) and maxima (blue) of the flow time course.

Comparison of flow time courses obtained in femoral artery (left) and femoral vein (right) in nine different heart cycle phases. Velocity distributions of the vessel (bottom line) illustrate heterogeneous flow distributions and turbulences, which are more pronounced in the artery.
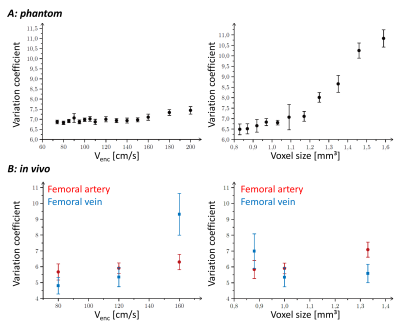
Variation coefficients for different venc factors and voxel sizes obtained in phantom (A) and in vivo (B) measurements.
DOI: https://doi.org/10.58530/2022/0793