0783
Identification of ’black hole’ T1 hypointense lesions using different intensity thresholds on gradient-echo T1w MRI1Danish Research Centre for Magnetic Resonance, Centre for Functional and Diagnostic Imaging and Research, Copenhagen University Hospital - Amager and Hvidovre, Hvidovre, Denmark, 2Neurology, Danish Multiple Sclerosis Center, Rigshospitalet, Glostrup, Denmark, 3Clinical Medicine, University of Copenhagen, Copenhagen, Denmark, 4Health Technology, Technical University of Denmark, Lyngby, Denmark, 5Neurology, Copenhagen University Hospital Bispebjerg, Copenhagen, Denmark
Synopsis
Black holes (BHs), T1 hypointense lesions, are known predictors of multiple sclerosis disease severity, however, their identification is strongly intensity dependent. As traditional spin-echo T1 scans are being replaced by 3D gradient-echo images, we need to understand how hypointense lesions on these images compare. We assessed whether an intensity threshold for MPRAGE images exists that would allow to match spin-echo black hole definitions. Simple thresholding was only able to reproduce black hole segmentations to an intermediate degree.
Introduction
So-called ‘black holes’ have been identified as a specific type of highly destructive lesions or white matter hyperintensities, which are T2 hyperintense and become hypointense on T1w images with time1. These are pathologically characterized by severe axonal and neuronal loss associated with chronic and irreversible tissue damage2-4. In multiple sclerosis (MS), black holes (BH) have been traditionally identified on spin-echo (SE) T1w images where their presence has been linked to clinical severity5,6. As 3D imaging has gained momentum, MPRAGE or other gradient-echo T1w images are more commonly acquired to allow for both tissue and lesion segmentation7. However, it is known that the level of lesional hypointensity differs strongly between SE and gradient-echo scans and that a large proportion of lesions on gradient-echo images appears ‘black hole’-like8,9. Here, we assessed whether a fixed intensity threshold may be identified across participants to match BH identification between gradient-echo (MPRAGE) and SE T1 scans.Methods
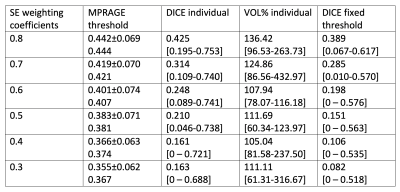
3T MRI data were collected in 25 relapsing-remitting MS patients enrolled in a non-inferiority trial of two MS treatments (NCT04688788), considering only baseline data prior to treatment initiation. 3D MPRAGE and 3D FLAIR images were collected prior to contrast administration, in addition to 2D SE T1w 8.5 min post-contrast. All images were acquired with 1x1 mm2 resolution in-plane, 3D scans 1 mm through-plane (sagittal), and 2D SE T1w with 3 mm axial slices (MPRAGE: TE/TR/TI=3.6/2700/1090 ms – SE T1: TE/TR=8.7/700 ms). First, the MPRAGE image was aligned to the MNI standard space, skull-stripped and bias field corrected. All other images were thereafter registered to the aligned MPRAGE image, skull-stripped and bias corrected (Ants, N4)10. A fully convolutional neuronal network learning architecture (FLEXCONN) was employed for automated segmentation of white matter lesions, using both FLAIR and. MPRAGE images11. Tissue segmentations from FAST (FSL library)12 were used to remove spurious lesional voxels outside of the white matter. All T1w image intensities were normalized to the image’s maximum intensity value. Similar to the approach by Tam et al.13, the subset of BHs among all detected T2 lesions was identified by determining an upper SE T1 intensity threshold for each lesion considering the local white matter intensity and cerebrospinal fluid signal across the image. Thereafter, lesion maps were thresholded at a series of MPRAGE T1 intensity values (first 10 intensity values between 0 and white matter, thereafter 50 values around the previously identified intensity corresponding to the maximum dice coefficient). The DICE similarity coefficient and volume difference relative to the reference spin-echo BH mask were computed to estimate the most appropriate T1 intensity threshold. Using the group’s mean T1 intensity threshold, the final MPRAGE BH masks were obtained. This procedure was followed using different intensity weights for the SE BH identification (varied between 0.3-0.8).Results
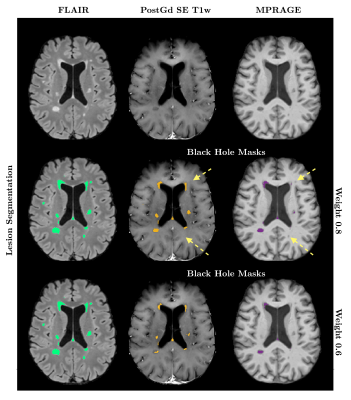
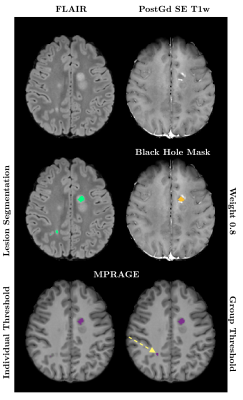
Using a weight of 0.8 in Tam et al.’s method, corresponding to an optimal agreement between manual BH identification and SE intensity thresholding, we found that the relative intensity on pre-contrast MPRAGE images yielded best agreement to the obtained SE BH masks when using a threshold of 0.44 ± 0.07. Nevertheless, the agreement between SE and gradient-echo BH estimations was moderate, with a median DICE score across the 25 subjects of 0.43 (ranging between 0.195-0.753). Even when using this optimal intensity threshold, MPRAGE images detected on average a 45% greater BH volume than SE scans. When modifying the intensity weight of the SE images to restrict ’black holeness’ definition to lower T1 intensities, thought to produce better correlation with clinical metrics, the DICE coefficient declined further (median 0.16). Results for all SE-weightings are summarized in Table 1 and Figure 1 compares segmentations with weights of 0.8 and 0.6 in one subject. When applying a common average intensity threshold to all subjects the median DICE across all subjects was reduced to 0.39 for the 0.8 weighting, as the average intensity threshold may be too low or too high for a given MPRAGE image. An example comparison of the effect of the individual and group thresholds is shown in Figure 2.Discussion and Conclusion
MPRAGE images are known to overestimate BH counts and volume compared to SE estimates. We showed that even when applying intensity thresholds on the MPRAGE images for BH identification, agreement with the traditional BH segmentations from SE T1w images remained moderate. Our data are in good agreement with a recent study at 1.5T, which also demonstrated over 40% volume difference between SE and gradient-echo scans8. Note that due to the cross-sectional nature of the current data, we could not establish whether the identified BHs were persistent BHs. More work is needed to understand how SE T1 images may be replaced by 3D gradient-echo scans in order to identify BHs in a similar fashion. However, it should also be evaluated which additional information MPRAGE data may provide to potentially identify lesional degrees of damage, and how T1 relaxation times may be used as supplementary information for the identification and monitoring of BHs14,15. Importantly, our data showed that the choice of T1 scan is still a relevant consideration at 3T for the estimation of BH, even if scans are now acquired at higher spatial resolution (both in 2D and 3D).Acknowledgements
Data for this study are acquired in multiple centres across Denmark and the study is supported by the Danish Regions (Regionernes Medicin- og Behandlingspulje). We wish to acknowledge that VW is supported by the Danish Sclerosefoundation (A40219/A41695) and the Lundbeckfoundation (R347-2020-2413). HL has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (grant agreement No 804746). HRS. holds a 5-year professorship in precision medicine at the Faculty of Health Sciences and Medicine, University of Copenhagen, sponsored by the Lundbeck Foundation [R186-2015-2138].
References
[1] van Waesberghe et al. Patterns of lesion development in multiple sclerosis: Longitudinal observations with T1-weighted spin-echo and magnetization transfer MR. AJNR Am J Neuroradiolog 1998;19:675-683.[2] Bitsch et al. A longitudinal MRI study of histopathologically defined hypointense multiple sclerosis lesions. Ann Neurol 2001;49:793-796.
[3] Brück et al. Inflammatory central nervous system demyelination: correlation of magnetic resonance imaging findings with lesion pathology. Ann Neurol 1997;42:783-793.
[4] van Waesberghe et al. Axonal loss in multiple sclerosis lesions: magnetic resonance imaging insights into substrates of disability. Ann Neurol 1999;46:747-754.
[5] Truyen et al. Accumulation of hypointense lesions (“black holes”) on T1 spin-echo MRI correlates with disease progression in multiple sclerosis. Neurology 1996;47(6):1469-1476.
[6] Paolillo et al. Brain atrophy in relapsing-remitting multiple sclerosis: relationship with ‘black holes’, disease duration and clinical disability. J Neurol Sci 2000;174(2):85-91.
[7] Valcarcel et al. A dual modeling approach to automatic segmentation of cerebral T2 hyperintensities and T1 black holes in multiple sclerosis. NeuroImage Clinical 2018;20:1211-1221.
[8] Dupuy et al. MRI detection of hypointense brain lesions in patients with multiple sclerosis: T1 spin-echo vs. gradient-echo. Eur J Radiol 2015;84:1564-1568.
[9] Lapucci et al. Degree of microstructural changes within T1-SE versus T1-GE hypointense lesions in multiple sclerosis: relevance for the definition of “black holes”. Eur Radiol 2020;30:3843-3851.
[10] Tustison et al. N4itk: improved n3 bias correction. IEEE Trans Med Imag 2010;29(3):1310-1320.
[11] Roy et al. Multiple sclerosis lesion segmentation from brain MRI via fully convoluted neural networks. 2018, Preprint arXiv:1803.09172.
[12] Zhang et al. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximation algorithm. IEEE Trans Med Imag 2001;20(1):45-47.
[13] Tam et al. The impact of intensity variations in T1-hypointense lesions on clinical correlations in multiple sclerosis. Mult Scler J 2011;17(8):949-957.
[14] Thaler et al. T1-thresholds in black holes increase clinical-radiological correlation in multiple sclerosis. PLoS One 2015;10(12):e0144693.
[15] Thaler et al. T1 recovery is predominantely found in black holes and is associated with clinical improvement in patients with multiple sclerosis. AJNR Am J Neuroradiol 2017;38(2):264-269.
Figures