0777
Improved Detection, Description and Efficiency of Multiple Sclerosis Lesions’ Assessment using a Dedicated Software1AI Medical, Zollikon, Switzerland, 2Stanford University, Stanford, CA, United States, 3University Zürich, Zürich, Switzerland
Synopsis
Yearly multiple sclerosis MRI follow-ups require the tedious and time-consuming manual comparing and counting of multiple demyelinating lesions, which can reach hundreds in some cases. We evaluated a semi-automatic software for the efficient assessment of such images, and found that a significant increase in the number of reported new multiple sclerosis lesions can be achieved in two-and-a-half minutes reading on average per case. In contrast, current standard reports missed 80% of the new lesions. In addition, The Jazz software permits the efficient reporting of slowly evolving lesions, an entity growing in clinical relevance and typically overlooked in current reporting.
Introduction
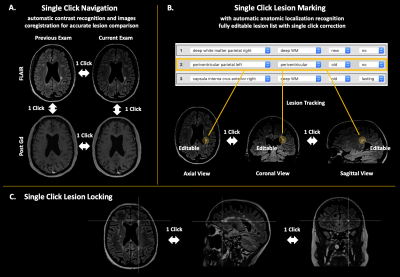
Multiple sclerosis (MS) is a widespread autoimmune, potentially disabling demyelinating disease of the central nervous system (CNS).1 MS patients require at least yearly follow-up controls with MRIs to evaluate disease evolution and possible treatment adjustment.2 These follow-ups involve the tedious and time-consuming manual comparing and counting of the patient’s demyelinating lesions, which requires to localize the lesions on all images and studies. The neuroradiologist needs to keep track of all lesions, either in her/his memory, or on a piece of paper, which is very error prone, for example in the event of an external interruption during the assessment. Because the number of focal lesions can reach hundreds in some severe cases, due to a lack of time, the neuroradiologist can be forced to compare grossly the lesion load, and some relevant evolution might remain unnoticed.Here, we evaluate the software Jazz3, a semi-automatic, deep-learning based software for the assessment of multiple sclerosis magnetic resonance images, wherein “semi-automatic” refers to the fact that each process gets verified by a radiologist. The software preprocesses the images before displaying them, including contrast recognition and coregistration of all images (of the current and of previous exams) to the current FLAIR, to optimize the reading time of the radiologist. The software permits efficient navigation, with single clicks, to the images of previous exams and between the images of the difference MRI sequences of each exam. Because all images are coregistered, this permits comparison of lesions in a very efficient way, “without eye movements”, maximizing efficiency and minimizing errors. (Fig 1) Jazz includes a semi-automatic lesion annotation tool, which permits saving of all relevant information in a lesion list using a single click. This permits the separation of the assessment of each lesion in a distinct step, again maximizing efficiency and minimizing errors (for example in case of external interruption during the reading). Corrections to lesions in the lesion list can be done with single clicks. (Fig 1) An editable report is then generated from the lesion list, including overview figures of the lesions, again saving time for the radiologist.
Methods
Forty multiple sclerosis follow-up examinations were collected, including 3D FLAIR, and pre- and post-Gad T1-weighted 3D images, and processed through the Jazz software. The corresponding report was saved as well. All data were fully anonymized. The cases were read by two experienced neuroradiologists (12 y and 7 y of experience), and reading time was recorded. The ground truth was defined by a second reading by the most experienced neuroradiologist, reevaluating all described lesions in all reports, using side-by-side comparison. Reported new lesions, slowly expending lesions (SELs), and gadolinium-enhancing lesions were compared on the FLAIR, using true positive, false negative, and total reported lesions.Results
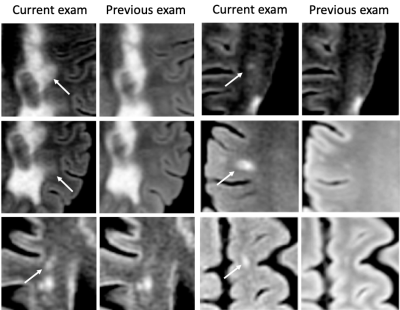
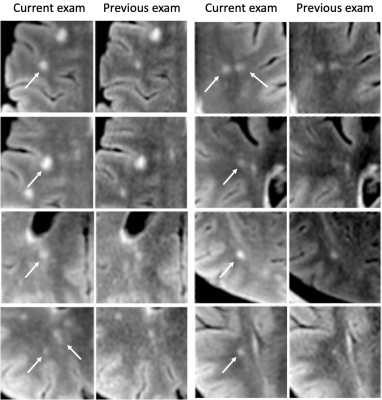
Reading using Jazz took 2 min 29 sec ± 2 min 15 sec per case for Reader 1, and 1 min 45 sec ± 51 sec for Reader 2. The ground truth reported 30 new lesions from 12 patients, and 44 SLEs from 13 patients. No gadolinium-enhancing lesions were described in any report. The standard report reported a total of 5 new lesions from 3 patients, missing 25 lesions from 11 patients. Using the Jazz software, Reader 1 detected 13 new lesions from 5 patients, and missed 17 lesions from 9 patients, while Reader 2 detected 25 new lesions from 12 patients, and missed 5 lesions from 3 patients. (Fig 2 and 3) The standard report reported no SLEs, while Reader 1 reported 41 SLEs from 12 patients, missing 3 lesions from 3 patients, while Reader 2 reported 7 SLEs from 5 patients, missing 37 SLEs from 12 patients. (Fig 2 and 4) Reader 2 reported 12 false positive lesions, while the standard report and Reader 1 both none.Discussion
We show that a significant larger number of new lesions are reported when using a dedicated semi-automatic software such as Jazz compared to the standard reporting method. The automation of multiple repetitive tasks, such as image contrast recognition, and an efficient navigations and lesion annotation tools frees the radiologist to concentrate on the lesion assessment, reducing the number of missed lesions. In addition, the reading with such a software is very time efficient, taking two-and-a-half minute on average, permitting significant productivity. This study also shows a significant number of slowly expending lesions reported with the Jazz software, unreported in the standard report. While some of those subtle modifications might be attributed to different acquisition technique, slowly expending lesions can be easily overlooked. The interest in their relevance has increased recently,4 as they are actively damaging nerve fibers, have been shown to be a marker of chronic active MS,5,6 and could be a specific target for treatment.Acknowledgements
No acknowledgement found.References
1. Howard J, Trevick S, Younger DS. Epidemiology of Multiple Sclerosis. Neurol Clin. 2016;34:919–939.
2. Miller DH, Grossman RI, Reingold SC, McFarland HF. The role of magnetic resonance techniques in understanding and managing multiple sclerosis. Brain. 1998;121 ( Pt 1):3–24.
3. Jazz, AI Medical AG; Accessed at: www.ai-medical.ch.
4. Calvi A, Haider L, Prados F, Tur C, Chard D, Barkhof F. In vivo imaging of chronic active lesions in multiple sclerosis. Mult Scler. Epub 2020 Sep 23.:135245852095858.
5. Elliott C, Wolinsky JS, Hauser SL, et al. Slowly expanding/evolving lesions as a magnetic resonance imaging marker of chronic active multiple sclerosis lesions. Mult Scler. 2019;25:1915–1925.
6. Elliott C, Belachew S, Wolinsky JS, et al. Chronic white matter lesion activity predicts clinical progression in primary progressive multiple sclerosis. Brain. 2019;142:2787–2799.
Figures