0769
Beyond DW-Based Analysis of Fiber Architecture: Estimating Orientation Distributions from High Angular Resolution Susceptibility Imaging1Max Planck Institute for Human Cognitive and Brain Sciences, Leipzig, Germany, 2Department of Electrical Engineering and Computer Sciences, UC Berkeley, Berkeley, CA, United States, 3Max Planck Institute for Evolutionary Anthropology, Leipzig, Germany, 4Tai Chimpanzee Project, Centre Suisse de Recherches Scientifiques en Cote d'Ivoire, Abidjan, Cote D'ivoire
Synopsis
We present a novel approach to estimate fiber Orientation Distribution Functions (ODFs) by applying the generalized Constant Solid Angle (CSA) method to High Angular Resolution Susceptibility Imaging (HARSI) data from post-mortem chimpanzee brain. The acquisition details and analytical pipelines are presented and derived susceptibility tensor metrics and ODFs are compared to metrics derived from traditional High Angular Resolution Diffusion Imaging (HARDI). The ODFs estimated from susceptibility data indicate comparable efficiency in resolving intersecting fiber orientations compared to HARDI-ODFs and increased sensitivity to secondary direction. This suggests a potential to obtain complementary information on brain white matter microstructural properties.
Introduction
Diffusion Tensor Imaging (DTI) is an established method for identifying the primary orientation of fiber bundles, based on the anisotropy of water diffusion. However, mono-tensorial measures cannot reliably estimate trajectories of intersecting fibers. In that regard, methods yielding orientation distribution functions (ODFs) show promising results.1 To retrieve information reflecting the complex fiber architecture in an imaging voxel, High Angular Resolution Diffusion-weighted Imaging (HARDI) has found a considerable role.2Anisotropy reflecting tissue microstructure is also inherent to the magnetic susceptibility—despite an entirely different physical mechanism.3,4 Consequently, Susceptibility Tensor Imaging (STI) based on the signal phase was shown to yield similar orientation information as DTI.5,6 Given such similarity, we may assume that STI shares the same shortcomings of DTI, that is, an incapability to resolve multiple fiber orientations.
Here, we present a phase-based method to extract robust ODFs employing the Constant Solid Angle (CSA) approach.7 Data acquisition in post-mortem primate brain is performed with High Angular Resolution Susceptibility-weighted Imaging (HARSI) and compared to ODF estimates obtained by HARDI. The new approach indicates promising identification of the peaks of multi-orientational fiber bundles to that resemble features of the HARDI-based results but demonstrate distinct differences that might yield complementary information on WM microstructure.
Methods
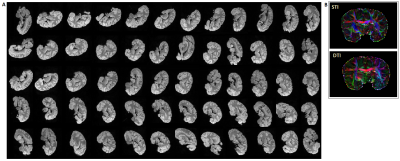
Complex 3D multi-echo (ME) GRE datasets (1mm isotropic nominal resolution; TE=3.5, … 45 ms; TE=30 ms selected for final analysis) were acquired with a 32-channel head-coil on a 3T MAGNETOM Skyra Connectom (Siemens, Erlangen Germany) from a post-mortem chimpanzee named ‘Fredy’ (male, 45years old, died from natural causes in Tai National Park, Ivory coast, brain exctraction interval 18h, preserved in paraformaldehyde in phosphate buffer saline (PBS)). The brain was cleaned of PBS, immersed in Fomblin and positioned in a 3D-printed container adapted to the individual anatomy (Figure 1). This container was centered in a spherical outer shell with angle indications to support robust reorientation of the specimen in the magnet (precision ≤3° for all axes). 60 independent orientations, computed employing an electrostatic repulsion model8, were sampled. ESPIRiT-SVD was used for coil combination.9,10Multi-orientation phase volumes were registered to a reference employing transformations that were derived by registering the corresponding magnitude volumes using FSL11. Laplacian phase unwrapping and background-phase removal using V-SHARP12 were performed on the registered phase volumes followed by iLSQR13 for susceptibility-tensor reconstruction. Diffusion-weighted (DW) 3D segmented ME-EPI data14 was acquired in the same specimen (1mm isotropic; TR = 104000 ms; TE = 53.5, …, 91 ms; b = 5000 s/mm2; 60 directions) and processed using FSL for eddy-current correction and diffusion-tensor reconstruction along with its eigen-analysis products.
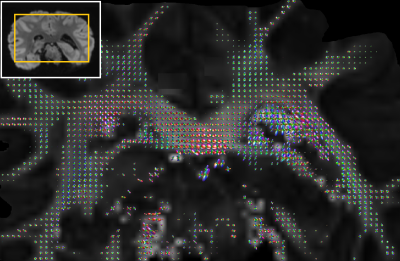
CSA-ODFs as implemented in the DIPY package was used for ODF analyses of HARDI and HARSI data. A schematic of the pipeline is presented in Figure 2. This analysis was restricted to a mask of sufficiently anisotropic WM [fractional-anisotropy (FA) threshold], after registration to a magnitude reference from the diffusion-weighed acquisitions. Outliers were excluded based on thresholding on the tissue phase and DW data.
Results
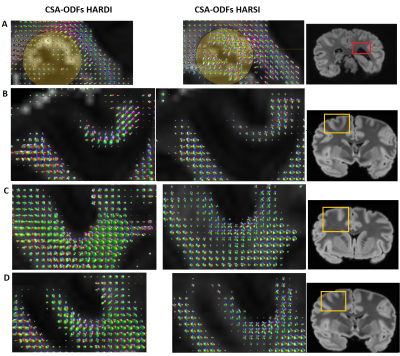
Figure 3A demonstrates the image quality obtained in 60 consecutive acquisitions at different orientation. Consistent registration results were achieved with the close-fitting anatomically shaped container and rotation device. Similarities of features extracted from susceptibility and diffusion tensors consistent with previous results5,15 (Figure 3B).CSA-ODF estimates from HARSI data resolved fiber crossings at angles >30-35° (Figure 4). Some additional noise is evident probably due to the multistep processing pipeline (susceptibility extraction/registrations/thresholding) as is an (expected) sensitivity of the susceptibility-based results to residual artifacts from air bubbles and border regions. Diffusion-based spherical harmonics appear of superior quality in such regions (Figures 3 and 4A).
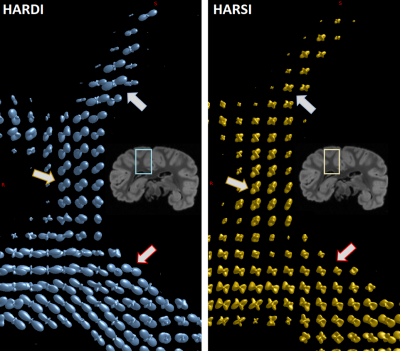
Besides a general similarity of the two estimations in resolving crossings, distinct differences in the shapes of the spherical harmonic distributions suggest a potentially superior SH peaks separation sensitivity of HARSI-ODFs in some regions. While HARDI-derived ODFs tended to have higher sensitivity towards a single direction, HARSI-derived ODFs indicate local multi-directional fibers more clearly, which is evident in Figures 4B-E and more clearly depicted in Figure 5.
Discussion
The ODFs estimated based on magnetic susceptibility acquired at high angular resolution indicate similarities with the existing gold standard approach, this is, HARDI-based ODF estimates. The comparison of complex fiber structures in selected ROIs suggest a potentially increased sensitivity in susceptibility-based ODF estimations, while diffusion-based ODFs point mostly towards the main orientation. This result is further corroborated by a comparison of STI and DTI primary eigenvector directions.Of note, HARSI acquisitions are restricted to investigations of fixed tissue specimens that can be freely rotated in the main magnetic field. Under such conditions, data can be acquired at very high spatial resolution (e.g., 300µm) without high demand on the gradient system. Moreover, signal loss during an echo time that achieves sufficient phase evolution is typically much smaller that loss due to T2 relaxation during the diffusion preparation. Susceptibility-based ODFs may, hence, yield complementary information on WM fiber architecture and achieve resolutions beyond current limits of diffusion-based acquisitions.
Acknowledgements
This work was funded by the EU through the ITN “INSPiRE-MED” (H2020-MSCA-ITN-2018, #813120).
Special thanks to the Evolution of Brain Connectivity (EBC) project, the Ministère de l’Enseignement Supérieur et de la Recherche Scientifique and theMinistère de Eaux et Fôrests in Côte d’Ivoire, and the Office Ivoirien des Parcs et Réserves for permitting the study, and the staff of the Taï ChimpanzeeProject.
References
1. Tournier J-D, Calamante F, Gadian DG, Connelly A. Direct estimation of the fiber orientation density function from diffusion-weighted MRI data using spherical deconvolution. NeuroImage. 2004;23:1176-1185.
2. Tuch DS, Reese TG, Wiegell MR, et al. High angular resolution diffusion imaging reveals intravoxel white matter fiber heterogeneity. Magn Reson Med. 2002;48:577-582.
3. Möller HE, Bossoni L, Connor JR, et al. Iron, myelin, and the brain: Neuroimaging meets neurobiology. Trends Neurosci. 2019;42(6):384-401.
4. Deistung A, Schweser F, Reichenbach JR. Overview of quantitative susceptibility mapping. NMR Biomed. 2017;30(4):e3569.
5. Liu C. Susceptibility tensor imaging. Magn Reson Med. 2010;63(6):1471-1477.
6. Li W, Liu C, Duong TQ, et al. Susceptibility tensor imaging (STI) of the brain. NMR Biomed. 2017;30(4):e3540.
7. Kamath A, Aganj I, Xu J, et al. Generalized constant solid angle ODF and optimal acquisition protocol for fiber orientation mapping. Proceedings of the MICCAI 2012 Workshop on Computational Diffusion MRI. Nice, France. 2012; 67-78.
8. Tournier J-D, Smith RE, Raffelt D et al. MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. NeuroImage. 2019;202:116137.
9. Uecker M, Lustig M. Estimating absolute-phase maps using ESPIRiT and virtual conjugate coils. Magn Reson Med. 2017;77(3):1201-1207.
10. Bilgic B, Polimeni JR, Wald LL, et al. Automated tissue phase and QSM estimation from multichannel data. Proceedings of the 24th Annual Meeting of ISMRM. Singapore 2016; 2849.
11. Jenkinson M, Beckmann CF, Behrens TE, et al. FSL. NeuroImage. 2012;62:782-790.
12. Özbay PS, Deistung A, Feng X, et al. A comprehensive numerical analysis of background phase correction with V-SHARP. NMR Biomed. 2017;30(4):e3550.
13. Li W, Wang N, Yu F, et al. A method for estimating and removing streaking artifacts in quantitative susceptibility mapping. NeuroImage. 2015;108:111-122.
14. Eichner C, Paquette M, Mildner T, et al. Increased sensitivity and signal-to-noise ratio in diffusion-weighted MRI using multi-echo acquisitions. NeuroImage. 2020;221:117172.
15. Gkotsoulias DG, Metere R, Su Y, et al. High angular resolution susceptibility and diffusion imaging in post mortem chimpanzee brain: Tensor characteristics and similarities. Proceedings of the 29th Annual Meeting of ISMRM. 2021; 3966.
Figures