0759
A Fast T2-weighted Approach for Prostate Imaging using non-Cartesian GRAPPA and Spiral TSE readout1Biomedical Engineering, Case Western Reserve University, CLEVELAND, OH, United States, 2Radiology, University of Michigan, Ann Arbor, MI, United States
Synopsis
In-gantry prostate biopsy relies on repeated time-consuming T2-weighted TSE imaging for needle guidance. Reducing the acquisition time for this scan has been challenging due to the spatial resolution and field-of-view requirements of these images, in addition to susceptibility and off resonance artifacts sources common to pelvic imaging. This work proposes a non-Cartesian GRAPPA approach with a short spiral in-out TSE readout to reduce acquisition time for needle guidance images. Comparisons of in vivo Cartesian and spiral TSE images with 300mm FOV, 1.17x1.17mm2 resolution, and 3mm slice thickness acquired in 61s and 8s respectively, are shown.
Introduction
MR-guided in-gantry prostate biopsy has been shown to be more sensitive and specific than other biopsy techniques1, but often entails long procedure time2. After initial high resolution planning images, repeated intermediate resolution T2-weighted turbo spin echo (TSE) images are obtained for introducer probe targeting the lesion of interest2. Acceleration of these guidance images would enable faster probe targeting during biopsy and improve clinical workflow. Parallel imaging is used to accelerate guidance images but is limited due to the intrusive appearance of ghost-like aliasing artifacts in Cartesian sampling. Spiral trajectories have been shown to reduce acquisition time3,4 and improve scan efficiency5 of TSE acquisitions due to their greater sampling efficiency within a single readout6, and have undersampling artifacts that manifest as blur rather than ghosts6. This work explores spiral TSE with non-Cartesian GRAPPA as a potential fast substitute for Cartesian TSE in prostate imaging.Methods
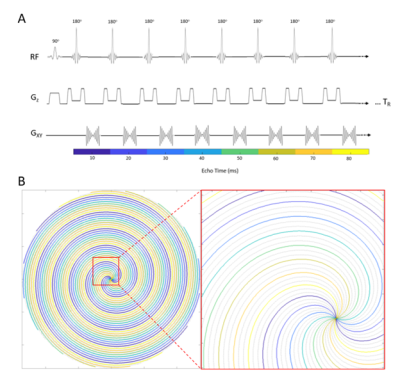
Uniform density spiral in-out trajectories were designed by concatenating a fully sampled spiral-out gradient waveform with its time-reversed spiral-in waveform. A 216-interleaf spiral with short readout (TADC = 3.71ms) was designed to minimize off-resonance artifacts, and was integrated into a spiral TSE acquisition with ETL=36, TEeff=153ms, TR=4000sPhantom and in vivo data were obtained in this IRB-approved study on a 3T Siemens Vida scanner. Both fully-sampled and undersampled (R=3) spiral TSE data were collected. Additionally, a second spiral image was collected to correct spiral TSE artifacts3 and any additional off resonance artifacts7 with the same imaging parameters, but reversed interleaf sampling order and time-reversed interleaf gradient waveforms. Undersampled data were reconstructed with spiral GRAPPA8 using a single calibration frame collected with ETL=36, TEeff=160ms, and TR=500ms. A 32x8 (read x proj) calibration segment was used to obtain enough kernel repetitions to determine the GRAPPA weights. For comparison, a Cartesian TSE image was acquired with 300mm2 FOV, 1.17x1.17mm2 resolution, 201Hz/px bandwidth, ETL=20, TEeff = 102ms, TR=4000s, and GRAPPA R=2 with integrated ACS lines.
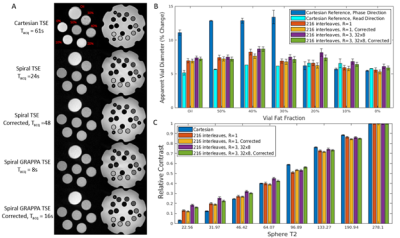
Phantom data were acquired using a 16-channnel head array to compare the apparent resolution and contrast of spiral TSE, accelerated spiral TSE, and clinically-used Cartesian TSE. A fat fraction phantom9 with vials containing 0%, 10%, 20%, 30%, 40%, 50% and 100% fat fraction was used to assess apparent resolution to observe impact of fat off resonance at different fat fraction regimes. Apparent resolution is defined by the % increase in vial diameter with the termination of the vial edge defined as a drop to 10% of mean vial signal. Contrast was assessed by computing the average signal of each vial normalized by the highest signal vial in the T2-array of the ISMRM/NIST MRI system phantom10. In vivo data were collected from four healthy volunteers using a 30-channel body array.
Results
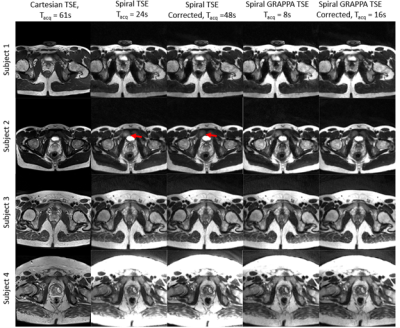
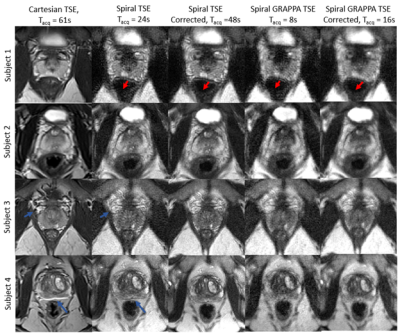
Figure 2 shows that spiral TSE variants had a maximum apparent vial size increase of 9.1% compared to 7.2% in the Cartesian readout direction and 14.4% in the Cartesian phase encoding direction, respectively, in high fat fraction vials. At low fat fraction (≤20%), the maximum increase in apparent vial size was 8.7%, 7.2%, and 6.8% respectively and was comparable between all sequences. The contrast for vials with different T2 values was similar between the spiral acquisitions and Cartesian TSE except in vials with T2 values of less than 45ms, where spiral TSE signal was hypointense compared with Cartesian TSE images.Figures 3 and 4 show in vivo images of the prostate collected using standard Cartesian TSE, Spiral TSE, and Spiral TSE with R=3 reconstructed with spiral GRAPPA. Spiral acquisition reduces chemical shift artifacts near the rectum and seminal vesicles (Figure 4). The acquisition of an additional spiral image for correction doubles acquisition time but also reduces artifacts around the phantom vials for all spiral TSE approaches (Figure 2), intensity artifacts near the bladder (Figure 3), and distortion artifact near the rectum (Figure 4), and recovers anatomic detail in the prostate (Figure 4).
Discussion
Fully sampled spiral TSE images can be acquired in 24s with increased noise compared to Cartesian TSE (Tacq=61s). Spiral TSE images with an acceleration factor of R=3 and non-Cartesian GRAPPA reconstruction can be collected in but suffer from increased blurring and noise enhancement. This issue could potentially be improved by using a lower TEeff, but necessitating a longer acquisition time or a long spiral readout with fewer interleaves that could enhance blur. The acquisition of additional spiral correction images reduces the appearance of artifacts in the ISMRM/NIST phantom and in vivo prostate images and reduces noise in both spiral TSE variants at the cost of acquisition speed. Further experiments in patients with pathology are necessary to assess lesion conspicuity using these approaches.Conclusion
Spiral TSE with non-Cartesian GRAPPA can be used to acquire T2-weighted images of the prostate in a fraction of the time as gold-standard Cartesian acquisitions (8 seconds vs. 61 seconds) with similar contrast properties but enhanced noise/blurring. Corrections can be applied to further reduce artifacts at the expense of additional scan time. The work presented here could help accelerate probe targeting during in-gantry prostate biopsy, though additional acceleration is needed for real-time image guidance.Acknowledgements
This work was supported by the NIH Grants: F30CA239355 and T32GM07250.References
1. Egbers N, Schwenke C, Maxeiner A, Teichgräber U, Franiel T. MRI-guided core needle biopsy of the prostate: acceptance and side effects. Diagn Interv Radiol. 2015;21(3):215-221. doi:10.5152/dir.2014.14372
2. Verma S, Choyke PL, Eberhardt SC, et al. The Current State of MR Imaging–targeted Biopsy Techniques for Detection of Prostate Cancer. Radiology. 2017;285(2):343-356. doi:10.1148/radiol.2017161684
3. Li Z, Karis JP, Pipe JG. A 2D spiral turbo-spin-echo technique. Magnetic Resonance in Medicine. 2018;80(5):1989-1996. doi:10.1002/mrm.27171
4. Li Z, Wang D, Robison RK, et al. Sliding-slab three-dimensional TSE imaging with a spiral-In/Out readout. Magn Reson Med. 2016;75(2):729-738. doi:10.1002/mrm.25660
5. Munsch F, Taso M, Zhao L, et al. Rotated spiral RARE for high spatial and temporal resolution volumetric arterial spin labeling acquisition. NeuroImage. 2020;223:117371. doi:10.1016/j.neuroimage.2020.117371
6. Wright KL, Hamilton JI, Griswold MA, Gulani V, Seiberlich N. Non-Cartesian Parallel Imaging Reconstruction. J Magn Reson Imaging. 2014;40(5):1022-1040. doi:10.1002/jmri.24521
7. Fielden SW, Meyer CH. A simple acquisition strategy to avoid off-resonance blurring in spiral imaging with redundant spiral-in/out k-space trajectories. Magn Reson Med. 2015;73(2):704-710. doi:10.1002/mrm.25172
8. Heidemann RM, Griswold MA, Seiberlich N, et al. Direct parallel image reconstructions for spiral trajectories using GRAPPA. Magn Reson Med. 2006;56(2):317-326. doi:10.1002/mrm.20951
9. Hines CDG, Yu H, Shimakawa A, McKenzie CA, Brittain JH, Reeder SB. T1 independent, T2* corrected MRI with accurate spectral modeling for quantification of fat: validation in a fat-water-SPIO phantom. J Magn Reson Imaging. 2009;30(5):1215-1222. doi:10.1002/jmri.21957
10. Russek SE, Boss M, Jackson EF, et al. Characterization of NIST/ISMRM MRI System Phantom. In: Proceedings of the International Society of Magnetic Resonance in Medicine. ; 2012:2456.
Figures