0758
Motion insensitive T2-weighted dual-echo steady-state (DESS) imaging of the liver using alternating readout gradients1Radiology, University of Wisconsin-Madison, Madison, WI, United States, 2Biomedical Engineering, University of Wisconsin-Madison, Madison, WI, United States, 3Medical Physics, University of Wisconsin-Madison, Madison, WI, United States, 4Emergency Medicine, University of Wisconsin-Madison, Madison, WI, United States, 5Medicine, University of Wisconsin-Madison, Madison, WI, United States
Synopsis
In this work, we present a novel method to reduce motion sensitivity for T2-weighted dual-echo steady-state (DESS) imaging, through the use of a readout alternation (ROA) approach. ROA can be achieved by inverting polarity of gradient pulses in the readout axis, alternating every TR. Bloch equation simulations that included small physiological motion in the liver, and volunteer liver imaging was performed using the proposed method. Simulation and volunteer studies demonstrated that ROA effectively suppresses signal loss and motion-related artifacts.
INTRODUCTION
Dual steady-state echo (DESS) imaging enables simultaneous fast and three-dimensional acquisitions of free induction decay (FID)- and refocused echo-signals(1). DESS is commonly used for cartilage imaging to obtain T2-weighted images(2) and T2 mapping(3).Since DESS creates an additional readout gradient moment to acquire the echo-signal, it is sensitive to motion. Severe motion artifacts are particularly problematic in the abdomen and chest due to physiological motion. Therefore, a very limited number of studies have investigated in DESS for body imaging applications(4).
In this study, we propose a method to reduce motion artifacts for T2 weighted DESS acquisitions, through the use of alternating polarity readout gradients.
THEORY
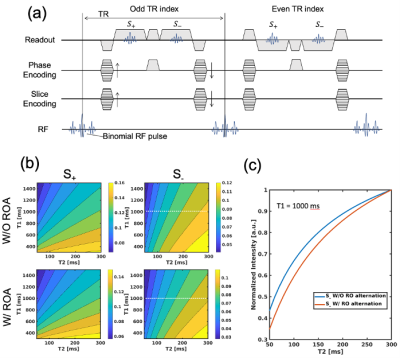
DESS is characterized by two consecutive readout gradients, which generate fast imaging with steady precession (FISP) and time-reversed FISP (PSIF) signals, denoted as S+ and S-. In this study, we introduce readout alternation (ROA) approach, which is achieved by inverting the polarity of the readout gradient pulses every TR as shown in Figure 1a.To achieve steady-state, an additional spoiler is inserted in phase-encoding direction. The proposed acquisition scheme has a different signal pathway from conventional DESS, results in a different signal response to T1/T2.
Figure 1b shows the simulated signal intensity of S+ and S- depending on T1 and T2 for conventional and the proposed DESS. The normalized intensities of S- signals shown in Figure 1c indicate that ROA gives slightly higher T2 sensitivity.
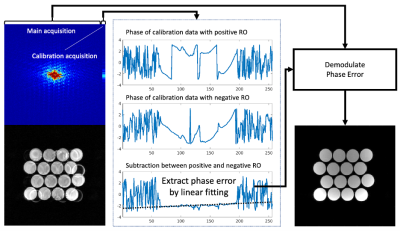
Phase correction (shown in Figure 2) is required to correct the errors caused by gradient delays from alternating polarity gradients. To calculate the phase error, calibration acquisitions without phase-encoding are performed after the main acquisitions. The phase error can be estimated by obtaining phase subtraction between even and odd numbers of phase-encoding lines. Signals can be demodulated using the estimated phase.
Motion and flow artifacts arise from a phase modulation by motion during readout gradient pulses. Assuming a constant velocity during TR, the phase modulation can be expressed as the below equations.
$$\phi_m =\int_0^\tau G\cdot r(t)dt\quad [1]$$
with
$$r(t)=\nu\tau+r_0\quad [2]$$
where G is a linear magnetic field generated by the gradient coil, r is the position of an isochromat, τ is a time duration spin experience the field, ν is the velocity of motion, and r0 is the initial position of the isochromat. Equations 1 and 2 lead to the following equation, which describes the modulated phase error for a specific TR index .
$$\phi_m (j)=\frac{1}{2}G\cdot \Delta\cdot \nu\cdot TR\cdot j^2+G\cdot \Delta\cdot \nu\cdot (\frac{1}{2}TR-\Delta)\cdot j\quad [3]$$
where Δ is the pulse duration. Motion causes linear/quadratic phase change in every TR, resulted in artifacts in image domain.
The magnitude of artifacts can be characterized by the sidelobe-to-peak ratio (SPR)(5) of the point spread function (PSF) defined as
$$SPR=max_{i\neq j} |\frac{PSF(i,j)}{PSF(i,i)}| \quad [4]$$
where i and j are the pixel position of the PSF.
METHODS
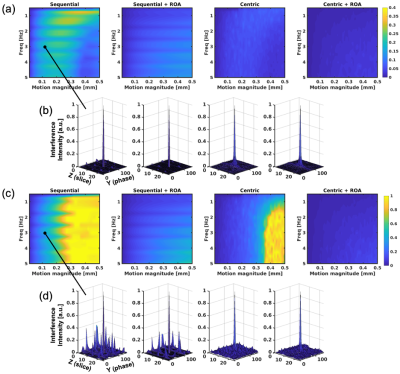
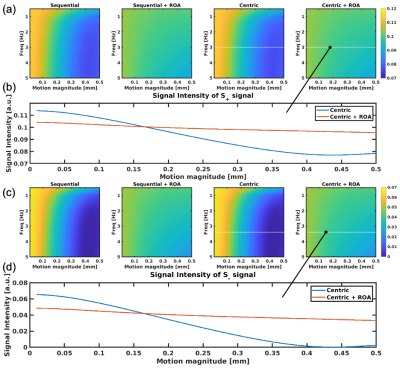
To evaluate motion artifacts in S+ and S- signals caused by motion, Bloch equation simulations were performed, including the effects of phase errors induced by motion. We assumed slow and small oscillating motions with frequency of 0 to 5Hz, and peak-to-peak motion magnitude of 0.1 to 0.5mm. Also, centric encoding, which is known as a motion robust approach(6), was compared with sequential encoding, and also used on conjunction with ROA. SPRs and signal intensities of S+ and S- for simulated signals were measured.A liver volunteer was included to demonstrate the feasibility and motion sensitivity of the proposed approach. The acquisition was performed with breath-holding on a clinical 3.0T MRI (Premier, GE Healthcare). To suppress fat signal, a binomial water-excitation pulse was used.
RESULTS
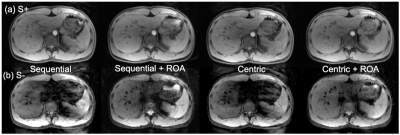
Figure 3 plots measured SPRs from simulations, suggesting that S- signal has higher sensitivity to motion, with severe artifacts in case of sequential ordering acquisitions. Importantly, ROA reduced signal losses due to motion, as shown in Figure 4, even with centric encoding. Figure 4b plots the signal intensities for 3Hz motion. As seen in this figure, the signal drops sharply without ROA as the magnitude of the motion increases.S+ and S- images of the liver of the healthy volunteer are shown in Figures 5a and 5b. Severe signal losses were observed in the images without ROA, and avoided with ROA. Centric encoding reduces artifacts further when compared to sequential encoding.
DISCUSSION
In this study, we performed simulation and volunteer studies to demonstrate a novel DESS imaging strategy using alternating readout gradients to mitigate motion. The results suggest that ROA improves artifacts and signal losses due to motion.ROA approach may be promising method for fast T2-weighted imaging of the liver. A small amount physiological motion during breath-holding may be the source of artifacts. This was notable in sever signal loss of the left lobe, likely due to cardiac motion. The volunteer study demonstrated that the combination of centric encoding and ROA improves both motion artifacts and signal losses.
A major limitation of ROA is slightly lower signal-to-noise ratio compared to conventional DESS. However, optimization of sequence may alleviate this limitation. More rigorous clinical evaluation including radiologist evaluation is also needed .
In conclusion, we have developed and presented a novel approach to reduce motion sensitivity of DESS. Sequence optimization and clinical evaluation will be performed in future work.
Acknowledgements
The authors wish to acknowledge support from GE Healthcare who provides research support to the University of Wisconsin. Finally, Dr. Reeder is a Romnes Faculty Fellow, and has received an award provided by the University of Wisconsin-Madison Office of the Vice Chancellor for Research and Graduate Education with funding from the Wisconsin Alumni Research Foundation.References
1. Hargreaves B. Rapid gradient-echo imaging. Journal of Magnetic Resonance Imaging 2012;36(6):1300-1313.
2. Hardy PA, Recht MP, Piraino D, Thomasson D. Optimization of a dual echo in the steady state (DESS) free-precession sequence for imaging cartilage. J Magn Reson Imaging 1996;6(2):329-335.
3. Sveinsson B, Chaudhari A, Gold G, Hargreaves B. A simple analytic method for estimating T2 in the knee from DESS. Magnetic resonance imaging 2017;38:63-70.
4. Dregely I, Margolis DA, Sung K, Zhou Z, Rangwala N, Raman SS, Wu HH. Rapid quantitative T2 mapping of the prostate using three‐dimensional dual echo steady state MRI at 3T. Magnetic Resonance in Medicine 2016;76(6):1720-1729.
5. Lustig M, Donoho D, Pauly JMJMRiMAOJotISfMRiM. Sparse MRI: The application of compressed sensing for rapid MR imaging. 2007;58(6):1182-1195.
6. Wilman AH, Riederer SJ. Performance of an elliptical centric view order for signal enhancement and motion artifact suppression in breath‐hold three‐dimensional gradient echo imaging. Magnetic resonance in medicine 1997;38(5):793-802.
Figures