0756
A rapid motion-corrected T2*-weighted multi-shot EPI technique for the Emergency Department setting1Neuroradiology, Barrow Neurological Institute, Phoenix, AZ, United States, 2Philips Healthcare, Gainesville, FL, United States
Synopsis
T2*w-MRI is routinely used for the diagnosis of hemorrhage and stroke in the Emergency Department, where time is critical and motion is often a concern. However, conventional T2*w-GRE have long scan times, and often suffer from motion artifacts. Parallel imaging and compressed sensing reduce the scan time, but are still prone to motion. ssEPI is fast and motion-insensitive, but suffers from severe geometric distortions. msEPI alleviates the distortion artifacts, but at the cost of increased motion sensitivity. This work presents an msEPI with a navigator echo for rapid motion-corrected T2*w-MRI. Volunteer and patient studies have demonstrated its robustness.
Introduction
MRI has become more common in the Emergency Department (ED).1 A majority of ED MRI scans are performed on the brain/head/neck and spine.2 In the ED setting, time is critical. In addition, ED patients often suffer from severe pain and cannot hold still during MRI scans. T2*w-MRI is an important tool to assess hemorrhage3 and stroke4 in ED patients. T2* contrast is achieved with gradient-echo (GRE) based sequences.5 Conventional GRE collects one line per shot in Cartesian k-space, resulting in long scan time and sensitivity to motion. Therefore, a rapid motion-robust technique is highly desired.Various strategies can be employed to accelerate the scans. Parallel imaging6,7 and compressed sensing8 can reduce the scan time but are still prone to motion. Single-shot EPI is fast and motion insensitive, but suffers from severe geometric distortions. Multi-shot EPI (msEPI) alleviates the distortion artifacts, but at the cost of increased motion sensitivity.
We propose an msEPI technique for rapid motion-corrected T2*w-MRI, inspired by recent progresses in motion correction for DW-EPI9-11. The proposed technique is assessed on volunteers and verified on patients in the ED setting.
Methods
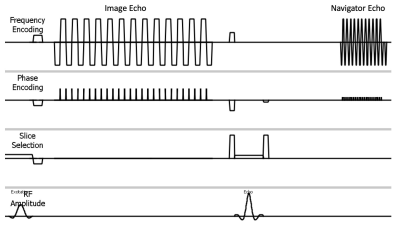
Figure 1 illustrates the sequence diagram. For each shot, the first EPI readout collects a portion of k-space of the image data; following the additional refocusing RF pulse, the second EPI readout acquires a low-resolution navigator image.Head motion introduces inter-shot geometric discrepancies, and B0 field variations that cause phase errors.12 These motion and phase errors are estimated from the navigator image using an approach adapted from a revised PROPELLER algorithm.13 To reconstruct the image, an iterative conjugate gradient method, similar to Ref 11, is used to solve:$$\it\begin{array}{c}argmin\\ \overrightarrow{x}\end{array}||SFC{\Phi}T\overrightarrow{x}-\overrightarrow{y} ||_2^2$$where $$$\overrightarrow{x}$$$ represents the corrected image, $$$\overrightarrow{y}$$$ is the measured k-space data, $$$T$$$ motion (in-plane rotation and translation) operator, $$$\it\Phi$$$ phase error map, $$$C$$$ coil sensitivity map, $$$F$$$ Fourier transform operator, and $$$S$$$ multi-shot sampling function.
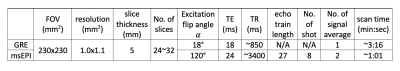
The pulse sequence was implemented on Philips Ingenia scanners. Due to the additional refocusing RF pulse, the T1-related tissue signal recovery is interrupted and the CSF-tissue contrast and gray-matter-to-white-matter (GM-WM) contrast are altered, compared to msEPI without a navigator echo. A set of excitation flip angles 𝛼 (70~140°) and TRs (2700~4500 ms) were simulated and assessed on volunteers to determine a protocol that preserves comparable tissue contrast to standard-of-care (SOC)-GRE. The selected imaging parameters for 1.5T are listed in Table 1. To investigate the performance of motion and phase correction, volunteers were instructed to 1) keep still, 2) rotate (mostly in-plane motion), 3) nod (mostly through-plane motion), and 4) rotate and nod (mixed motion) the head. To evaluate the image quality in the clinical environment, over 20 patients were scanned. All scans were approved by the institutional review board.
Results
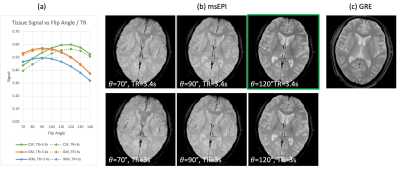
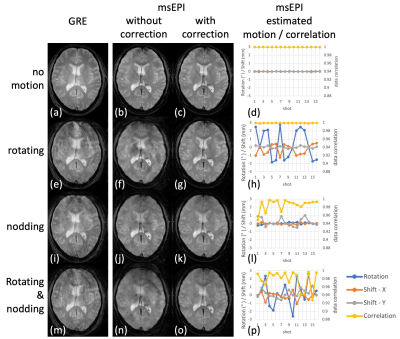
Fig. 2a shows the simulated signals. Good CSF-tissue contrast is achieved with (𝛼 > 110°, TR = 3.4 s) or (𝛼 > 120°, TR = 3 s). Higher flip angles increase CSF-tissue contrast, while reducing GM and WM signal and consequently SNR. Longer TRs increase CSF signal while gaining little GM or WM signal, with increased scan time. Fig. 2b shows volunteer images from a few combinations, among which (𝛼 = 120°, TR = 3.4 s) produces comparable contrast to SOC-GRE (Fig. 2c).Fig. 3 illustrates the benefit of the proposed motion and phase correction method on volunteers. Artifacts are observed in GRE (column 1) in the presence of motion. msEPI are shown without (column 2) and with correction (column 3), along with the estimated motions (column 4). Head rotations (row 2) introduce mostly in-plane rotation and translation (Fig. 3h), with artifacts in msEPI (Fig. 3f) being well corrected (Fig. 3g). Head nodding (row 3) results in mostly through-plane motion, which was not directly quantized but indirectly reflected by the correlation coefficients (Fig. 3l, yellow curve); these artifacts (Fig. 3j) were not fully corrected but substantially reduced (Fig. 3k). Mixed rotating and nodding (row 4) resulted in both in/through-plane motion (Fig. 3p) where motion-induced artifacts (Fig. 3n) were again significantly reduced (Fig. 3o).
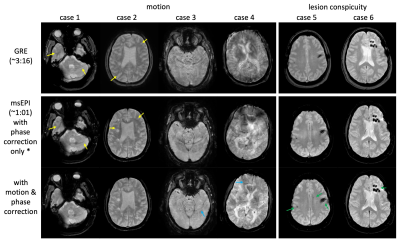
Fig. 4 demonstrates motion robustness in the clinical environment. The left panel shows representative patient cases with different amounts of motion, where msEPI with the proposed correction results in significantly reduced motion artifact in all cases. Residual artifacts, if present, manifest as a few isolated streaks that are readily recognized and in general are not destructive. The right panel demonstrates comparable lesion conspicuity between msEPI and SOC-GRE.
Discussion and Conclusion
By acquiring data from multiple EPI shots, the proposed T2*w-msEPI achieves a balance between scan speed and geometric distortion artifacts. With an iterative reconstruction approach using motion/phase information estimated from navigator data via an adapted PROPELLER algorithm, it has excellent performance in correcting for in-plane motion and phase errors. Unlike PROPELLER, it is not straightforward to incorporate a data weighting strategy to mitigate through-plane artifacts. Nonetheless, the iterative reconstruction process improves inter-shot data consistency and alleviates through-plane artifacts.In summary, the proposed msEPI technique is ~67% faster than SOC-GRE, provides additional motion-compensation capability, while preserving lesion conspicuity and tissue contrast. Clinical results demonstrate its feasibility as an alternative to SOC-GRE for T2*w-MRI in the ED.
Acknowledgements
The authors thank Dr. Guruprasad Krishnamoorthy and Philips Healthcare for their research support.
References
1. Quaday KA, Salzman JG, Gordon BD. Magnetic resonance imaging and computed tomography utilization trends in an academic ED. Am J Emerg Med. 2014;32(6):524-528. doi:10.1016/j.ajem.2014.01.054
2. Sánchez Y, Yun BJ, Prabhakar AM, et al. Magnetic Resonance Imaging Utilization in an Emergency Department Observation Unit. West J Emerg Med. 2017;18(5):780-784. doi:10.5811/westjem.2017.6.339923.
3. Yuan M-K, Lai P-H, Chen J-Y, et al. Detection of subarachnoid hemorrhage at acute and subacute/chronic stages: comparison of four magnetic resonance imaging pulse sequences and computed tomography. J Chin Med Assoc. 2005;68(3):131-137. doi:10.1016/S1726-4901(09)70234-5
4. Morita N, Harada M, Uno M, et al. Ischemic Findings of T2*-Weighted 3-Tesla MRI in Acute Stroke Patients. Cerebrovasc Dis. 2008;26(4):367-375. doi:10.1159/000151640
5. Chavhan GB, Babyn PS, Thomas B, Shroff MM, Haacke EM. Principles, techniques, and applications of T2*-based MR imaging and its special applications. Radiographics. 2009;29(5):1433-1449. doi:10.1148/rg.295095034
6. Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: sensitivity encoding for fast MRI. Magn Reson Med. 1999;42(5):952-962.
7. Griswold MA, Jakob PM, Heidemann RM, et al. Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magn Reson Med. 2002;47(6):1202-1210. doi:10.1002/mrm.10171
8. Lustig M, Donoho D, Pauly JM. Sparse MRI: The application of compressed sensing for rapid MR imaging. Magn Reson Med. 2007;58(6):1182-1195. doi:10.1002/mrm.21391
9. Jeong H-K, Gore JC, Anderson AW. High-resolution human diffusion tensor imaging using 2-D navigated multishot SENSE EPI at 7 T. Magn Reson Med. 2013;69(3):793-802. doi:10.1002/mrm.24320
10. Guhaniyogi S, Chu M-L, Chang H-C, Song AW, Chen N-K. Motion immune diffusion imaging using augmented MUSE for high-resolution multi-shot EPI. Magn Reson Med. 2016;75(2):639-652. doi:10.1002/mrm.25624
11. Steinhoff M, Mertins A, Bornert P. SENSE-based multi-shot DWI reconstruction with extra-navigated rigid motion and contrast correction for brain EPI. In: Proceeding of the 2020 ISMRM & SMRT Virtual Conference. ; 2020:4339.
12. Liu J, de Zwart JA, van Gelderen P, Murphy-Boesch J, Duyn JH. Effect of head motion on MRI B(0) field distribution. Magn Reson Med. 2018;80(6):2538-2548. doi:10.1002/mrm.27339
13. Pipe JG, Gibbs WN, Li Z, Karis JP, Schar M, Zwart NR. Revised motion estimation algorithm for PROPELLER MRI. Magn Reson Med. 2014;72(2):430-437. doi:10.1002/mrm.24929
Figures