0751
Rotational invariants of the cumulant expansion (RICE)1Center for Advanced Imaging Innovation and Research (CAI2R), Department of Radiology, New York University, School of Medicine, New York, NY, United States
Synopsis
We studied the symmetries of the diffusion and covariance tensors, both accessible through a general b-tensor acquisition. We showed that there is an unexplored contrast in the non-symmetric part of the covariance tensor and use it to compute the size-shape covariance of compartmental diffusion tensors. We measure all rotational invariants of the cumulant tensors in a normal volunteer, explore the novel size-shape contrast, and provide the relations between these invariants and typical diffusion contrasts.
Introduction
The diffusion MRI (dMRI) signal can be mathematically described via the cumulant expansion, yielding the commonly used DTI and DKI methods. Conventional linear tensor encoding (LTE) allows measuring the kurtosis tensor $$$\boldsymbol{W}$$$1 with 15 independent components. However, dMRI protocols adding free (beyond LTE) $$$q$$$-space trajectories2 enable probing a more general diffusion covariance tensor $$$\boldsymbol{C}$$$3 with 21 independent components. Here we characterize and explore the information content of $$$\boldsymbol{C}$$$. We represent the $$$6=21-15$$$ extra parameters in terms of a rank-2 symmetric tensor $$$\boldsymbol{A}$$$, and construct its rotational invariants $$$A_0$$$ and $$$A_2$$$. While $$$A_0$$$ contributes to microscopic fractional anisotropy ($$$\mu$$$FA), the previously unexplored invariant $$$A_2$$$ yields the size-shape covariance of the compartmental diffusivities over a voxel. We measure all rotational invariants of $$$\boldsymbol{W}$$$ and $$$\boldsymbol{A}$$$ in a normal volunteer, explore the novel size-shape contrast, and show how to calculate axial and radial kurtosis metrics fast, without projecting the tensors onto a principal fiber direction.Theory
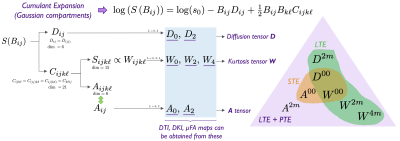
Cumulant expansion. If a voxel contains multiple Gaussian compartments, the signal from any q-space trajectory, $$$\boldsymbol{q}(t)$$$, is fully determined by a b-tensor $$$\boldsymbol{B}$$$3:$$\begin{aligned}S&=s_0\sum_\alpha\,f_\alpha\,e^{-B_{ij}D_{ij}^\alpha},\qquad(1)\\B_{ij}&=\int_0^{\text{TE}}\!\!q_i(t)\,q_j(t)\,\mathrm{d}t,\qquad(2)\end{aligned}$$where $$$D_{ij}^\alpha$$$ is the diffusion tensor of compartment $$$\alpha$$$ and $$$b=B_{ii}=\mathrm{tr}\,\boldsymbol{B}$$$ is the b-value (Einstein's convention of summation over repeated indices assumed).We can write the cumulant expansion of Eq. 1 up to $$$\mathcal{O}(B^2)$$$:$$\log(S)=\log(s_0)-B_{ij}\,D_{ij}+\tfrac12B_{ij}B_{k\ell}C_{ijk\ell},\qquad(3)$$where $$$\boldsymbol{D}$$$ and $$$\boldsymbol{C}$$$ are the diffusion and covariance tensors, determined by:$$D_{ij}=\langle\,\!D_{ij}^\alpha\rangle,\,\,\,C_{ijk\ell}=\langle\,\!D_{ij}^\alpha\,D_{k\ell}^\alpha\rangle-\langle\,\!D_{ij}^\alpha\rangle\,\langle\,\!D_{k\ell}^\alpha\rangle,\qquad(4)$$with angular brackets denoting averages over the distribution of $$$D_{ij}^\alpha$$$ in a voxel. $$$\boldsymbol{C}$$$ has minor and major symmetries and can be decomposed into its symmetric part, $$$\boldsymbol{S}$$$, and its complement, $$$\boldsymbol{A}$$$:4
$$\begin{aligned}C_{ijk\ell}&=S_{ijk\ell}+A_{ijk\ell},\\S_{ijk\ell}&=C_{(ijk\ell)}=\tfrac13(C_{ijk\ell}+2C_{i\ell\,\!kj}),\quad\quad\quad\quad(5)\\A_{ijk\ell}&=C_{ijk\ell}-C_{(ijk\ell)}=\tfrac23(C_{ijk\ell}-C_{i\ell\,\!kj}).\end{aligned}$$Here symmetrization over tensor indices between (...) is assumed.
Tensor $$$\boldsymbol{S}$$$ is accessible with LTE, it has 15 degrees of freedom (dof) and is proportional to the kurtosis tensor: $$$W_{ijk\ell}=\frac{3}{\overline{D}^2}C_{(ijk\ell)}=\frac{3}{\overline{D}^2}S_{ijk\ell}$$$, where $$$\overline{D}$$$ is mean diffusivity.
Tensor $$$\boldsymbol{A}$$$, inaccessible with LTE, has 6 dof which can be mapped to a rank-2 symmetric tensor4:$$\begin{aligned}A_{mn}&=\epsilon_{ikm}\epsilon_{j\ell\,\!n}A_{ijk\ell}=\delta_{mn}(A_{iikk}-A_{ikik})+2A_{mknk}-2A_{mnkk}=\delta_{mn}(C_{iikk}-C_{ikik})+2C_{mknk}-2C_{mnkk},\qquad(6)\\A_{ijk\ell}&=\tfrac16(\epsilon_{ikm}\epsilon_{j\ell\,\!n}+\epsilon_{i\ell\,\!m}\epsilon_{jkn})A_{mn},\end{aligned}$$where $$$\delta_{ij}$$$ is Kronecker's delta, and $$$\epsilon_{ijk}$$$ the Levi-Civita symbol. Spherical tensor encoding (STE) is sensitive to the isotropic part of $$$\boldsymbol{A}$$$ while planar tensor encoding (PTE) probes the full $$$\boldsymbol{A}$$$ tensor. Figure 1 shows a diagram of this decomposition.
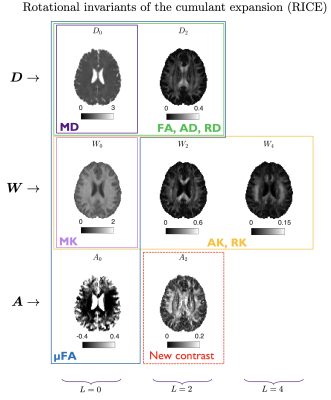
STF decompositions. We represent voxel-wise tensors $$$\boldsymbol{D}$$$, $$$\boldsymbol{S}$$$ and $$$\boldsymbol{A}$$$ in STF basis $$$\mathcal{Y}_{i_1...i_\ell}^{\ell\,\!m}$$$5:$$\begin{aligned}D_{ij}&=D_{00}\tfrac{1}{\sqrt{4\pi}}\delta_{ij}+D_{2m}\mathcal{Y}_{ij}^{2m},\\S_{ijk\ell}&=S_{00}\tfrac{1}{\sqrt{4\pi}}\delta_{(ij}\delta_{k\ell)}+S_{2m}\mathcal{Y}_{(ij}^{2m}\delta_{k\ell)}+S_{4m}\mathcal{Y}_{ijk\ell}^{4m},\qquad(7)\\A_{ij}&=A_{00}\tfrac{1}{\sqrt{4\pi}}\delta_{ij}+A_{2m}\mathcal{Y}_{ij}^{2m}.\end{aligned}$$This basis is analogous to spherical harmonics. For every order $$$\ell$$$ we can define rotational invariants6,7,8, e.g. $$$S_\ell=\sqrt{\frac{\sum_m|S_{\ell\,\!m}|^2}{4\pi(2\ell+1)}}$$$.
Writing compartmental diffusion tensors in STF basis, $$$D_{ij}^\alpha=D_{00}^\alpha\tfrac{1}{\sqrt{4\pi}}\delta_{ij}+D_{2m}^\alpha\mathcal{Y}_{ij}^{2m}$$$, we derive all terms in Equation 7 averaged over arbitrary distribution of compartmental $$$D_{ij}^\alpha$$$:$$L=0\quad\left\{\begin{aligned}D_0=&\,\langle\,\!D_0^\alpha\rangle\\S_0=&\,\langle(D_0^\alpha)^2\rangle-D_0^2+5\big(\,\langle(D_2^\alpha)^2\rangle-D_2^2\big)\qquad(8)\\A_0=&\,2\big(\,\langle(D_0^\alpha)^2\rangle-D_0^2\big)-\tfrac{25}{2}\big(\,\langle(D_2^\alpha)^2\rangle-D_2^2\big)\end{aligned}\right.$$ $$L=2\quad\left\{\begin{aligned}D_{2m}=&\,\langle\,\!D_{2m}^\alpha\rangle\\S_{2m}=&\,2\,(\,\langle\,\!D_0^\alpha\,D_{2m}^\alpha\rangle-D_0\,D_{2m})+\tfrac{8}{21C_2^2}\,(\langle\,\!D_{2m'}^\alpha\,D_{2n'}^\alpha\rangle-D_{2m'}\,D_{2n'})\,\mathcal{Y}^{2m'}_{ij}\,\mathcal{Y}^{2n'}_{jk}\mathcal{Y}^{2m}_{ki}\qquad(9)\\A_{2m}=&\,-2\,(\,\langle\,\!D_0^\alpha\,D_{2m}^\alpha\rangle-D_0\,D_{2m})+\tfrac{4}{3C_2^2}\,(\langle\,\!D_{2m'}^\alpha\,D_{2n'}^\alpha\rangle-D_{2m'}\,D_{2n'})\,\mathcal{Y}^{2m'}_{ij}\,\mathcal{Y}^{2n'}_{jk}\mathcal{Y}^{2m}_{ki}\end{aligned}\right.$$ $$L=4\quad\left\{\begin{aligned}S_{4m}=&\,\tfrac{8}{35C_4^2}\,(\langle\,\!D_{2m'}^\alpha\,D_{2n'}^\alpha\rangle-D_{2m'}\,D_{2n'})\,\mathcal{Y}^{4m}_{ijk\ell}\,\mathcal{Y}^{2m'}_{(ij}\mathcal{Y}^{2n'}_{k\ell)}.\qquad(10)\end{aligned}\right.$$
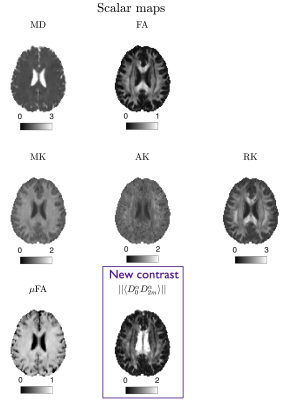
Conventional contrasts. We can derive DTI maps from RICE:$$\begin{aligned}\text{MD}&=\overline{D}=\tfrac{1}{4\pi}\int_{\mathbb{S}^2}d\hat{\boldsymbol{n}}D(\hat{\boldsymbol{n}})=\tfrac13\delta_{ij}D_{ij}=\tfrac{1}{\sqrt{4\pi}}D_{00}=D_0,\qquad(11)\\\text{FA}^2&=\frac32\frac{{V_{\lambda}(\boldsymbol{D})}}{{V_{\lambda}(\boldsymbol{D})}+3\overline{D}^{2}}=\frac{75D_2^2}{4D_0^2+50D_2^2},\qquad(12)\end{aligned}$$where $$$V_{\lambda}(\boldsymbol{D})$$$ is the variance of the eigenvalues of $$$\boldsymbol{D}$$$. For kurtosis, we use tensor derived metrics rather than average apparent kurtosis6,9,10:$$\text{MK}=\overline{W}=\tfrac{1}{4\pi}\int_{\mathbb{S}^2}d\hat{\boldsymbol{n}}W(\hat{\boldsymbol{n}})=\tfrac15\delta_{ij}\delta_{k\ell}W_{ijk\ell}=\tfrac{1}{\sqrt{4\pi}}W_{00}=W_0.\qquad(13)$$Often a main fiber population is assumed, thus, $$$\boldsymbol{D}$$$ and $$$\boldsymbol{W}$$$ can be projected to this principal axis $$$\hat{\boldsymbol{v}}_1$$$:$$\begin{aligned}\text{AD}&=D_\|=D(\hat{\boldsymbol{v}}_1),\quad &&\text{RD}=D_\perp=\tfrac{1}{2\pi}\int_{\mathbb{S}^2}d\hat{\boldsymbol{n}}D(\hat{\boldsymbol{n}})\delta(\hat{\boldsymbol{n}}\cdot\hat{\boldsymbol{v}}_1),\qquad(14)\\\text{AK}&=W_\|=\frac{\overline{D}^2}{D_\|^2}\,W(\hat{\boldsymbol{v}}_1),&&\text{RK}=W_\perp=\frac{\overline{D}^2}{D_\perp^2}\,\tfrac{1}{2\pi}\int_{\mathbb{S}^2}d\hat{\boldsymbol{n}}W(\hat{\boldsymbol{n}})\delta(\hat{\boldsymbol{n}}\cdot\hat{\boldsymbol{v}}_1).\end{aligned}$$Note that if $$$\boldsymbol{D}$$$ and $$$\boldsymbol{W}$$$ possess axial symmetry, we can compute the above maps without projecting onto the fiber basis but directly from RICE maps:$$\begin{aligned}\text{AD}_\text{ax,sym}&=D_0+5D_2,\quad&&\text{RD}_\text{ax,sym}=D_0-\tfrac52D_2\qquad\qquad\qquad\qquad(15)\\\text{AK}_\text{ax,sym}&=\frac{\overline{D}^2}{D_\|^2}\,(W_0+5W_2+9W_4),\quad&&\text{RK}_\text{ax,sym}=\frac{\overline{D}^2}{D_\perp^2}\,(W_0-\tfrac52W_2+\tfrac{27}{8}W_4).\end{aligned}$$Extracting the shape variance, $$$\langle(D_2^\alpha)^2\rangle$$$, we can compute $$$\mu$$$FA3,11
$$\mu\text{FA}^{2}=\frac32\frac{\langle\,\!V_{\lambda}(\boldsymbol{D})\rangle}{\langle\,\!V_{\lambda}(\boldsymbol{D})\rangle+3\overline{D}^{2}}=\frac{75\langle(D_2^\alpha)^2\rangle}{4D_{0}^{2}+50\langle(D_2^\alpha)^2\rangle}=\frac{60(S_0-\tfrac12A_0)+675D_2^2}{40(S_0-\tfrac12A_0)+450D_2^2+36D_0^2}.\qquad(16)$$
Novel contrast. All maps above are independent of $$$A_{2m}$$$ elements, which contain previously unexplored information. Following Eq. 9, we can obtain size-shape covariances and compute an invariant that is complementary to DTI-DKI-$$$\mu$$$FA:$$\langle\,\!D_0^\alpha\,D_{2m}^\alpha\rangle=\tfrac{7}{18}S_{2m}-\tfrac19A_{2m}+D_0D_{2m},\quad\rightarrow\quad||\langle\,\!D_0^\alpha\,D_{2m}^\alpha\rangle||=\sqrt{\sum_{m=-2}^{2}\langle\,\!D_0^\alpha\,D_{2m}^\alpha\rangle^2}.\qquad(17)$$
Experiments
After providing inform consent, a 30yo female volunteer underwent MRI in a whole body 3T-system (Siemens Healthcare, Prisma) using a 20-channel head coil. Maxwell-compensated free gradient diffusion waveforms were used to yield linear, planar, and spherical b-tensor encoding using a prototype spin echo sequence with EPI readout12. Linear b-tensors (30 directions $$$b=1ms/\mu\,\!m^2$$$ + 60 directions $$$b=2ms/\mu\,\!m^2$$$) and planar b-tensors (60 directions $$$b=1.5ms/\mu\,\!m^2$$$) were acquired. Imaging parameters: voxel size$$$\,=2\times2\times2\,$$$mm$$$^3$$$, $$$T_R=4.2s$$$, $$$T_E=90$$$ms, bandwidth=1818Hz/Px, $$$R_\text{GRAPPA}=2$$$, partial Fourier$$$\,=6/8$$$, multiband$$$\,=2$$$. Total scan was time 11.5 minutes.Results
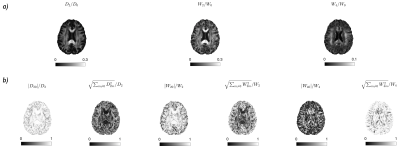
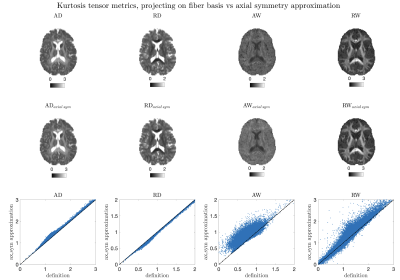
All rotational invariants of the cumulant expansion (RICE) maps are shown in Fig. 2. Figure 3 shows conventional diffusion contrasts together with a $$$||\langle\,\!D_0^\alpha\,D_{2m}^\alpha\rangle||$$$ map that contains the proposed size-shape covariance. The relative contributions from $$$L=0,2,4$$$ sectors in $$$\boldsymbol{D}$$$ and $$$\boldsymbol{W}$$$ are analyzed and shown in Fig. 4. Furthermore, we rotate each voxel and separate the contributions of the main axis vs the rest ($$$m=0\,\text{vs}\,m\neq0$$$ at the fiber coordinate frame). Finally, a comparison between fiber-basis projection maps (axial and radial diffusion/kurtosis) and axial symmetry approximations is shown in Fig. 5.Discussion and Conclusion
We classified the symmetries of the full diffusion covariance tensor $$$\boldsymbol{C}$$$ accessible under a general diffusion tensor-encoding acquisition. We showed that there is an unexplored contrast in the non-symmetric part of $$$\boldsymbol{C}$$$, and related it to the size-shape covariance of compartmental diffusion tensors. The RICE maps belong to distinct irreducible representations of rotations and thereby represent the ``orthogonal" contrasts up to $$$B^2$$$. This mutual independence may provide improved sensitivity to detect independent changes in disease and development.Acknowledgements
This work has been supported by NIH under NINDS award R01 NS088040 and NIBIB awards R01 EB027075 and P41 EB017183. The authors are grateful to Sune N. Jespersen for fruitful discussions.References
1. J. H. Jensen, J. A. Helpern, A. Ramani, H. Lu, and K. Kaczynski, “Diffusional Kurtosis Imaging: The quantifica- tion of non-gaussian water diffusion by means of magnetic resonance imaging,” Magnetic Resonance in Medicine, vol. 53, pp. 1432–1440, 2005.
2. D. Topgaard, “Multidimensional diffusion mri,” Journal of Magnetic Resonance, vol. 275, pp. 98–113, 2017.
3. C.-F.Westin,H.Knutsson,O.Pasternak,F.Szczepankiewicz,E.O ̈zarslan,D.vanWesten,C.Mattisson,M.Bo- gren, L. J. O’Donnell, M. Kubicki, D. Topgaard, and M. Nilsson, “q-space trajectory imaging for multidimensional diffusion MRI of the human brain,” NeuroImage, vol. 135, pp. 345–362, 2016.
4. Y. Itin and F. W. Hehl, “Irreducible decompositions of the elasticity tensor under the linear and orthogonal groups and their physical consequences,” in Journal of Physics: Conference Series, vol. 597, IOP Publishing, 2015.
5. K. S. Thorne, “Multipole expansions of gravitational radiation,” Rev. Mod. Phys., vol. 52, pp. 299–339, 1980.
6. H. Lu, J. Jensen, A. Ramani, and J. Helpern, “Three-dimensional characterization of non-gaussian water diffusion in humans using diffusion kurtosis imaging,” NMR in Biomedicine, vol. 19, no. 2, pp. 236–247, 2006.
7. M. Reisert, E. Kellner, B. Dhital, J. Hennig, and V. G. Kiselev, “Disentangling micro from mesostructure by diffusion MRI: A Bayesian approach,” NeuroImage, vol. 147, pp. 964 – 975, 2017.
8. D. S. Novikov, J. Veraart, I. O. Jelescu, and E. Fieremans, “Rotationally-invariant mapping of scalar and orien- tational metrics of neuronal microstructure with diffusion MRI,” NeuroImage, vol. 174, pp. 518 – 538, 2018.
9. B. Hansen, T. E. Lund, R. Sangill, and S. N. Jespersen, “Experimentally and computationally fast method for estimation of a mean kurtosis,” Magnetic Resonance in Medicine, vol. 69, no. 6, pp. 1754–1760, 2013.
10. S. N. Jespersen, J. L. Olesen, B. Hansen, and N. Shemesh, “Diffusion time dependence of microstructural param- eters in fixed spinal cord,” NeuroImage, vol. 182, pp. 329–342, 2017.
11. F. Szczepankiewicz, D. van Westen, E. Englund, C.-F. Westin, F. St ̊ahlberg, J. L ̈att, P. C. Sundgren, and M. Nilsson, “The link between diffusion MRI and tumor heterogeneity: Mapping cell eccentricity and density by diffusional variance decomposition (DIVIDE),” NeuroImage, vol. 142, pp. 522–532, 2016.
12. F. Szczepankiewicz, C.-F. Westin, and M. Nilsson, “Maxwell-compensated design of asymmetric gradient wave- forms for tensor-valued diffusion encoding,” Magnetic Resonance in Medicine, vol. 0, pp. 1–14, 2019.
Figures