0745
Prediction of survival in paediatric brain tumour patients using multicentre leakage-corrected perfusion MRI
Stephanie Withey1,2,3, Lesley MacPherson4, Adam Oates4, Stephen Powell3, Jan Novak2,3,5, Laurence Abernethy6, Barry Pizer7, Richard Grundy8, Paul S. Morgan8,9,10, Simon Bailey11, Dipayan Mitra12, Theodoros N. Arvanitis2,13, Dorothee P. Auer10,14,15, Shivaram Avula6, and Andrew C. Peet2,3
1RRPPS, University of Birmingham NHS Foundation Trust, Birmingham, United Kingdom, 2Oncology, Birmingham Women’s and Children’s NHS Foundation Trust, Birmingham, United Kingdom, 3Institute of Cancer and Genomic Sciences, University of Birmingham, Birmingham, United Kingdom, 4Radiology, Birmingham Women’s and Children’s NHS Foundation Trust, Birmingham, United Kingdom, 5Department of Psychology, Aston University, Birmingham, United Kingdom, 6Radiology, Alder Hey Children’s NHS Foundation Trust, Liverpool, United Kingdom, 7Oncology, Alder Hey Children’s NHS Foundation Trust, Liverpool, United Kingdom, 8The Children’s Brain Tumour Research Centre, University of Nottingham, Nottingham, United Kingdom, 9Medical Physics, Nottingham University Hospitals, Nottingham, United Kingdom, 10Division of Clinical Neuroscience, University of Nottingham, Nottingham, United Kingdom, 11Sir James Spence Institute of Child Health, Royal Victoria Infirmary, Newcastle upon Tyne, United Kingdom, 12Neuroradiology, Royal Victoria Infirmary, Newcastle upon Tyne, United Kingdom, 13Institute of Digital Healthcare, University of Warwick, Coventry, United Kingdom, 14Nottingham University Hospitals Trust, Neuroradiology, Nottingham, United Kingdom, 15NIHR Nottingham Biomedical Research Centre, Nottingham, United Kingdom
1RRPPS, University of Birmingham NHS Foundation Trust, Birmingham, United Kingdom, 2Oncology, Birmingham Women’s and Children’s NHS Foundation Trust, Birmingham, United Kingdom, 3Institute of Cancer and Genomic Sciences, University of Birmingham, Birmingham, United Kingdom, 4Radiology, Birmingham Women’s and Children’s NHS Foundation Trust, Birmingham, United Kingdom, 5Department of Psychology, Aston University, Birmingham, United Kingdom, 6Radiology, Alder Hey Children’s NHS Foundation Trust, Liverpool, United Kingdom, 7Oncology, Alder Hey Children’s NHS Foundation Trust, Liverpool, United Kingdom, 8The Children’s Brain Tumour Research Centre, University of Nottingham, Nottingham, United Kingdom, 9Medical Physics, Nottingham University Hospitals, Nottingham, United Kingdom, 10Division of Clinical Neuroscience, University of Nottingham, Nottingham, United Kingdom, 11Sir James Spence Institute of Child Health, Royal Victoria Infirmary, Newcastle upon Tyne, United Kingdom, 12Neuroradiology, Royal Victoria Infirmary, Newcastle upon Tyne, United Kingdom, 13Institute of Digital Healthcare, University of Warwick, Coventry, United Kingdom, 14Nottingham University Hospitals Trust, Neuroradiology, Nottingham, United Kingdom, 15NIHR Nottingham Biomedical Research Centre, Nottingham, United Kingdom
Synopsis
Dynamic susceptibility (DSC-) MRI provides measures of relative cerebral blood volume (rCBV) in paediatric brain tumours. Correction of rCBV for the effects of contrast agent leakage can be done using post-processing techniques. Eighty-five patients with a range of paediatric brain tumours underwent DSC-MRI scans at 4 centres using variable protocols. Leakage-corrected and uncorrected DSC-MRI parameters were calculated and patients were followed up. Median DSC-MRI parameters significantly predicted overall survival.
Introduction
Paediatric brain tumour survival rates vary between tumour types and with grade1. Early identification of those at higher risk may allow better treatment decisions to be made. Conventional descriptors of paediatric brain tumour survival include tumour type and grade, which rely on histopathology being obtained following surgery / biopsy2. This is invasive and may not be available due to tumour location or results being delayed. Dynamic susceptibility-contrast (DSC-) MRI involves rapid scanning following injection of a contrast agent and provides non-invasive estimates of perfusion parameters such as relative cerebral blood volume (rCBV). Leakage of contrast agent occurs in low-grade tumours resulting in under- or overestimation of rCBV. We have previously shown that leakage correction is essential in this patient group3. The aim of this work was to assess whether leakage-corrected DSC-MRI parameters could predict survival in paediatric brain tumour patients.Methods
85 patients underwent pre-treatment DSC-MRI scans at 4 centres on 6 different scanners. Scanning protocols were variable (Table 1). Pixel-by-pixel contrast agent concentration time courses were analysed using the Boxerman model of leakage correction4. Estimates of uncorrected and leakage-corrected rCBV (rCBVuncorr and rCBVcorr, respectively) and the leakage parameter, K2, were obtained. Patients subsequently underwent surgery / biopsy and tumours were classified and graded. Further treatment was undertaken according to the clinical needs of the patient. Patients were followed up locally. Kaplan Meier survival analysis was performed on patient characteristics including sex, tumour type, grade and DSC-MRI parameters.| Centre | Scanner | B0 | Sequence | TR / TE (ms) | TE (ms) | Slice thickness (mm) | No. slices | No. dyn. | Matrix | Pre-bolus? | n |
| 1 | Siemens | 1.5T | GE-EPI | 1490–1643 | 40 | 5.0 | 19–21 | 60 | 96 x 96 | Y | 8 |
| 1 | Philips | 3T | GE-EPI | 1830–1865 | 40 | 3.5 | 30 | 60 | 96 x 96 | Y | 3 |
| 2 | Siemens | 3T | GE-EPI | 1570 | 29 | 3.5 | 16 | 60 | 64 x 64 | N | 12 |
| 3 | Philips | 3T | GE-EPI | 1666–2343 | 40 | 4.0 | 25-35 | 40 | 128 x 128 | Y | 26 |
| 4 | Philips | 1.5T | sPRESTO | 16.7-17.2 | 25 | 3.5 | 30-36 | 60 | 64-80 x 64-80 | Y (n=12) N (n=15) n/k (n=9) | 15 |
| 4 | Philips | 3T | sPRESTO | 15.5-16.0 | 24 | 3.5 | 30–34 | 60 | 128 x 128 | 16 | |
| 4 | Philips | 3T | GE-EPI | 582-1866 | 18.4-40 | 3.5-7.0 | 30 | 40-60 | 96 x 96 | 5 |
Results
Median follow-up time was 88 months (range = 45 – 183 months). At analysis, 20 patients had died, 65 were alive (Table 2). Figure 1 shows example parameter maps from two patients with high-grade tumours. Survival differed between the two patients and was predicted better by median DSC-MRI parameters than by either tumour diagnosis or grade. Across the population, low rCBVuncorr and rCBVcorr were associated with significantly better survival (p<0.001 and 0.004, respectively) using cut-offs of 2.5 and 2.1, respectively. A positive K2, associated with leakage correction in low-grade tumours, was predictive of significantly better overall survival (p=0.020). Other prognostic factors were tumour type (p<0.01), tumour grade (p<0.01). Tumour volume, age, sex and centre were not significantly associated with survival.| Parameter | All | Alive | Dead | p-value |
| n | 85 | 65 | 20 | |
| Median survival (mths) | 70.2 | 88.1 | 22.2 | <0.001** |
| Tumour vol (cm3) | 28.1 ± 26.6 | 25.7 ± 25.9 | 36.1 ± 28.0 | <0.001** |
| rCBVuncorr | 1.19 ± 4.41 | 0.65 ± 4.64 | 2.94 ± 3.05 | <0.001** |
| rCBVcorr | 2.13 ± 1.56 | 1.90 ± 1.31 | 2.90 ± 2.05 | <0.001** |
| K2 | 0.009 ± 0.036 | 0.013 ± 0.039 | -0.003 ± 0.022 | 0.05 |
Discussion
Patients with well-perfused tumours prior to treatment had significantly worse overall survival than poorly-perfused tumours. rCBVuncorr was the best parameter at predicting survival. The leakage parameter, K2, was also significantly predictive of survival with positive K2 associated with better overall long-term survival. High perfusion is indicative of angiogenesis known to be important for tumour growth and results agree with those presented in other studies5,6. The significance of DSC-MRI parameters as predictors of survival is in line with conventional survival predictors, such as tumour type and grade. DSC-MRI parameters are available immediately after imaging and are obtained non-invasively. They may be particularly useful for survival prediction in tumours where histological diagnosis may be unavailable or delayed, allowing identification of patients whose prognosis is poor and who may benefit from additional treatments.Conclusion
In summary, perfusion MRI at diagnosis can aid in predicting which patients are likely to have better survival rates. Low perfusion was associated with better survival. This finding is robust across multiple centres, despite using multiple DSC-MRI protocols.Acknowledgements
Funded by: CRUK, EPSRC, MRC, NIHR, BCHRF, HHHOReferences
1National Cancer Registration and Analysis Service http://www.ncin.org.uk/cancer_type_and_topic_specific_work/cancer_type_specific_work/cancer_in_children_teenagers_and_young_adults/ Accessed 2021; 2Manias K, et al. Eur J Cancer (2017), 72: 251-65; 3Withey S, et al. Ped Radiol (In press); 4Boxerman JL, et al. AJNR (2006), 27(4): 859-67; 5Hipp, Neuro Oncol (2011), 13(8): 904-9; 6Tensaouti F, et al. Br J Radiol (2016), 89(1066): 20160537.Figures
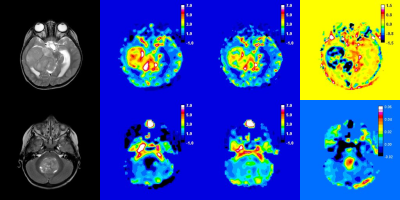
T2-w
image, rCBVuncorr, rCBVcorr and K2 maps from 2
high-grade tumour patients: (top) Glioblastoma with rCBVuncorr = 3.66,
rCBVcorr = 2.68 and K2 = -0.013. This patient died at 2.2
yrs following diagnosis; (bottom) A medulloblastoma with rCBVuncorr
= 0.67, rCBVcorr = 1.71 and K2 = 0.007. This patient was
alive at 11.4 yrs.

Kaplan-Meier plots showing the relationship between overall survival and
whole-tumour DSC-MRI parameters: a) median rCBVuncorr, b) median
rCBVcorr and c) K2. P-values from a log-rank test are
shown.
DOI: https://doi.org/10.58530/2022/0745