0740
Improved acquisition efficiency in T2-weighted fetal MRI with optimized variable flip angles and prospective wave-encoding1Massachusetts Institute of Technology, Cambridge, MA, United States, 2Fetal-Neonatal Neuroimaging and Developmental Science Center, Boston Children’s Hospital, Boston, MA, United States, 3Harvard Medical School, Boston, MA, United States, 4Athinoula A. Martinos Center for Biomedical Imaging, Charlestown, MA, United States, 5Department of Radiology, Harvard Medical School, Boston, MA, United States, 6Harvard-MIT Health Sciences and Technology, Cambridge, MA, United States, 7Institute for Medical Engineering and Science, Cambridge, MA, United States
Synopsis
Fetal MRI utilizes Half-Fourier-acquisition-single-shot-turbo-spin-echo (HASTE) for motion robust imaging. However, specific-absorption-rate (SAR) constraints from the refocusing pulse train lengthens scan time and increases vulnerability to motion-induced artifacts. Variable refocusing flip angle (VFA) acquisitions improve efficiency, but may suffer from poor contrast-to-noise-ratios (CNR). We propose an optimization technique for VFA that retains ~90% CNR with 2.5x SAR reduction. Furthermore, we demonstrate the first application of wave-encoding in fetal MRI at Rin-plane = 3-fold prospectively under-sampled acquisitions. Combining HASTE, VFA, and wave-encoding improves acquisition time with reduced repetition times and shorter echo-trains and could enable simultaneous-multislice acquisitions for further acceleration.
Introduction
Fetal MRI utilizes Half-Fourier-acquisition-single-shot-turbo-spin-echo (HASTE) to encode a slice in ~600 ms1, but the train of refocusing pulses induce ~1.5 second repetition times (TRs) between slices due to specific-absorption-rate (SAR) constraints. A previous heuristic variable flip angle (VFA) train enabled $$$TR \approx 600$$$ ms with 2.9x SAR reduction but compromised CNR in pregnancy2-4, while simulations explored wave-encoding for further acquisition efficiency5-8.We propose an optimization scheme9,10 to design VFA trains with improved contrast-to-noise (CNR) ratios in comparison to the previous heuristic design2. Simulations, phantom, and in-vivo acquisitions compare the SNR, CNR, and SAR of the optimized VFA train. We also implement prospective wave-encoding in the HASTE sequence6,7 and, to our knowledge, demonstrate the first feasibility experiment of combining HASTE, VFA, and wave-encoding in pregnancy to improve acquisition efficiency.
Methods
Standard clinical HASTE at our site acquires 92 $$$k_y$$$ lines (511 ms readout) with 63% partial fourier, $$$TE$$$ = 122 ms, 24 auto-calibration lines, $$$R_{in-plane}$$$ = 2, and 160 degree refocusing pulses.We utilize an optimization scheme that designs the VFA control angles to maximize CNR10. Let $$$\alpha = [\alpha_1, \alpha_2, \alpha_3]$$$ be the first three control angles to design ($$$a_{final}$$$ = 30 degrees). Let $$$C_{T_2^A}(\alpha,t)$$$ denote signal evolution for tissue A with $$$T_2 = T_2^A$$$ generated by a VFA train specified with control angles $$$\alpha$$$. Posing the optimization problem:
$$\alpha^* = argmax_{\alpha} ||C_{T_2^A}(\alpha,TE) - C_{T_2^B}(\alpha,TE)||_2^2$$
designs control angles $$$\alpha^*$$$ that maximize contrast between two $$$T_2$$$-values, $$$T_2^A$$$ and $$$T_2^B$$$, at a specified echo-time. We use $$$T_2^A$$$ = 80 ms and $$$T_2^B$$$ = 120 ms and match the clinical HASTE acquisition with $$$TE$$$ = 122 ms. The optimization problem is solved with gradient descent using finite-differences to compute gradients.
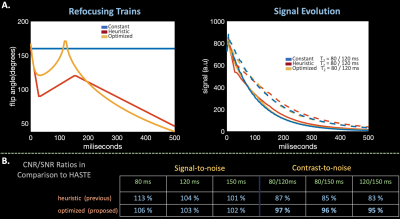
Figure 1.A compares constant 160 degree, previous heuristic, and optimized proposed refocusing trains and resultant signal evolutions from an isochromat based Bloch, Carr-Purcell-Meiboom-Gill simulation with slice-profile effects using characteristic fetal $$$T_2$$$ values11,12. Assuming signal strength and differences at $$$TE$$$ = 112 ms directly correlate with SNR and CNR, the optimized train achieves ~10% additional CNR in comparison to the previous heuristic regime and retains similar SNR.
Experiments
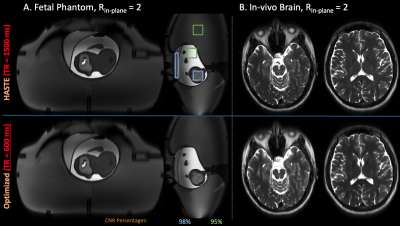
We acquired data with constant flip angles and optimized VFA using the standard HASTE acquisition (24 calibration lines, $$$R_{in-plane}$$$ = 2, 63% partial fourier, $$$TE$$$ = 122 ms) on an anthropomorphic fetal phantom13 (FOV = 350 x 350 mm, RES = 1.4 x 1.4 mm, slice-thickness = 3 mm) and adult brain (RES = 1.2 x 1.2 mm, slice-thickness = 3 mm).Second, standard HASTE data with constant flip angles and optimized VFA was acquired from a pregnant mother (FOV = 320 x 320 mm, RES = 1.25 x 1.25 mm, slice-thickness = 3 mm).
We compare product reconstructions of the above acquisitions, and compare CNR by computing ratios between regions-of-interest in the phantom.
Next, we implemented wave-encoding along the phase-encoded axis in the HASTE sequence5,14 and acquired prospective wave-encoded data with constant flip angles and optimized VFA on a pregnant mother ($$$R_{in-plane}$$$ = 3, 70% partial fourier, $$$TE$$$ = 122 ms, FOV = 320 x 320 mm, RES = 1.25 x 1.25 mm, slice-thickness = 3 mm, and wave trajectories with max-gradient-amplitude = 13.7 mT/m, max-slew-rate = 180 T/m/s, and 3 cycles)
Combining $$$R_{in-plane}$$$ = 3 with VFA improves SAR reduction from 2.5x to 2.75x since the sequence applies fewer refocusing pulses. For some subjects the minimum prescribed TR with VFA operates below the SAR limit, enabling simultaneous-multislice (SMS) encoding to further improve sequence efficiency.
As proof-of-concept, we generated retrospective wave-encoded-SMS k-space ($$$R_{in-plane}$$$ = 3, SMS = 2, slice-gap = 60 mm) from a prospectively wave-encoded acquisition with constant flip angles and optimized VFA on an adult brain ($$$R_{in-plane}$$$ = 3, 77% partial fourier, $$$TE$$$ = 122 ms, FOV = 224 x 204 mm, RES = 1 x 1 mm, slice-thickness = 3 mm, and wave trajectories with max-gradient-amplitude = 14.1 mT/m, max-slew-rate = 180 T/m/s, and 3 cycles). Note that prospectively, SAR constraints preclude SMS acquisitions with constant refocusing trains.
We reconstruct wave-encoded data with the wave-forward model and zero-filled partial fourier using an external GRE scan for coil estimation15 and a recently proposed wave-point-spread-function calibration technique16.
Results
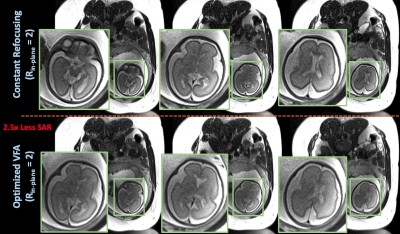
Figure 2 compares product brain and phantom reconstructions using standard HASTE with constant and optimized VFA. VFA yields qualitatively similar images, ~90% retained CNR, and 2.5x TR reduction.Figure 3 compares product HASTE in-pregnancy with constant flip angles and optimized VFA. Optimized VFA achieves similar image quality with 2.5x less SAR.
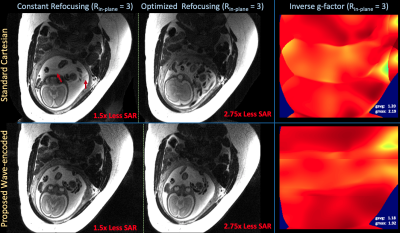
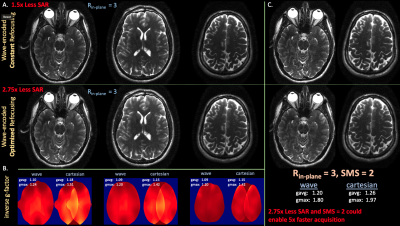
Figure 4 displays reconstructed prospective wave-encoded-HASTE fetal data with artifact reduction and improved inverse-gfactor maps in comparison to Cartesian acquisitions. VFA with $$$R_{in-plane}$$$ = 3 achieves 2.7x reduced SAR.
Figure 5 (a) illustrates reconstructions and inverse-g-factor maps of prospective brain wave-encoded-HASTE data with constant and optimized refocusing. Wave-encoding with VFA enables the potential SMS reconstructions in Figure 5(b).
Discussion
With the SAR decrease provided by VFA, future work will build on our adult-brain experiments and implement phase and partition wave-encoding with SMS in fetal MRI. Combining optimized VFA, wave, and SMS could reduce total volumetric scan times from ~1 minute to ~12-15 seconds, which we aim to deploy in larger cohorts of pregnant subjects to evaluate the incurred imaging tradeoffs.Acknowledgements
This work was supported in part by research grants NIH R01 EB017337, U01 HD087211, R01HD100009, R01 EB028797, U01 EB025162, P41 EB030006, U01 EB026996, R03EB031175 and the NVidia Corporation for computing support. In addition, this material is based upon work supported by the National Science Foundation Graduate Research Fellowship Program under Grant No. 1122374. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation.References
- Patel, M. R., Klufas, R. A., Alberico, R. A. & Edelman, R. R. Half-fourier acquisition single-shot turbo spin-echo (HASTE) MR: comparison with fast spin-echo MR in diseases of the brain. AJNR Am. J. Neuroradiol. 18, 1635–1640 (1997).
- Arefeen, Y. et al. Rapid fetal HASTE imaging using variable flip angles and simultaneous multislice wave-LORAKS.
- Loening, A. M. et al. Increased speed and image quality in single-shot fast spin echo imaging via variable refocusing flip angles. J. Magn. Reson. Imaging 42, 1747–1758 (2015).
- Busse, R. F., Hariharan, H., Vu, A. & Brittain, J. H. Fast spin echo sequences with very long echo trains: design of variable refocusing flip angle schedules and generation of clinical T2 contrast. Magn. Reson. Med. 55, 1030–1037 (2006).
- Bilgic, B. et al. Wave-CAIPI for highly accelerated 3D imaging. Magn. Reson. Med. 73, 2152–2162 (2015).
- Chen, F. et al. Autocalibrating motion-corrected wave-encoding for highly accelerated free-breathing abdominal MRI. Magn. Reson. Med. 78, 1757–1766 (2017).
- Chen, F. et al. Data‐driven self‐calibration and reconstruction for non‐cartesian wave‐encoded single‐shot fast spin echo using deep learning. J. Magn. Reson. Imaging 6, 698 (2019).
- Setsompop, K. et al. Blipped-controlled aliasing in parallel imaging for simultaneous multislice echo planar imaging with reduced g-factor penalty. Magn. Reson. Med. 67, 1210–1224 (2012).
- Keerthivasan, M. B. et al. An optimized single-shot sequence for fast T2w imaging of the brain. in Proceedings of the Joint Annual Meeting International Society for Magnetic Resonance in Medicine and the European Society for Magnetic Resonance in Medicine and Biology 16–21 (2018).
- Keerthivasan, M. B. et al. Abdominal T2-weighted imaging and T2 mapping using a variable flip angle radial turbo spin-echo technique. J. Magn. Reson. Imaging (2021) doi:10.1002/jmri.27825.
- Ben-Eliezer, N., Sodickson, D. K. & Block, K. T. Rapid and accurate T2mapping from multi-spin-echo data using Bloch-simulation-based reconstruction. Magnetic Resonance in Medicine vol. 73 809–817 (2015).
- Jeffrey N Stout, Congyu Liao, Esra Abaci Turk, Borjan Gagoski, P. Ellen Grant, Lawrence L. Wald, and Elfar Adalsteinsson. T1 and T2 mapping of the placenta and fetal brain with magnetic resonance fingerprinting (MRF) during maternal hyperoxia. In Proceedings of the 27th Annual Meeting of ISMRM 1182.
- Pablo Garcia-Polo, Borjan Gagoski, Bastien Gurrein, Eric Gale, Elfar Adalsteinsson, Ellen Grant, Lawrence Wald. An anthropomorphic MR phantom of the gravid abdomen including the uterus, placenta, fetus and fetal brain. In Proceedings of ISMRM 2015 1545 (2015).
- Chen, F. et al. Self-Calibrating Wave-Encoded Variable-Density Single-Shot Fast Spin Echo Imaging. Journal of Magnetic Resonance Imaging vol. 47 954–966 (2018).
- Tamir, J. I., Ong, F., Cheng, J. Y., Uecker, M. & Lustig, M. Generalized magnetic resonance image reconstruction using the Berkeley Advanced Reconstruction Toolbox. in ISMRM Workshop on Data Sampling & Image Reconstruction, Sedona, AZ (2016).
- Iyer, S. et al. Wave-encoding and Shuffling Enables Rapid Time Resolved Structural Imaging. arXiv [physics.med-ph] (2021).
Figures