0737
Optimisation of Structural MR Imaging Sequences in a Neonatal Cohort at 64 mT1Centre for the Developing Brain, King's College London, London, United Kingdom, 2Neonatal Intensive Care Unit, Guy's & St Thomas' Hospital NHS Foundation Trust, London, United Kingdom, 3Medical Physics, Guy's & St Thomas' Hospital NHS Foundation Trust, London, United Kingdom, 4Centre for Neuroimaging Sciences, King's College London, London, United Kingdom, 5Hyperfine, Inc., Guildford, CT, United States
Synopsis
Point-of-care Magnetic Resonance scanning is a novel and potentially transformative technology, utilising Ultra-Low Field permanent magnets to facilitate bedside neuroimaging. Through iterative T2w sequence optimisation, we demonstrate the feasibility of using ultra-low field portable 64mT MR scanning in neonates. This pilot data demonstrates that images can provide sufficient contrast for tissue differentiation and identify pathological lesions. Scanning with ongoing intensive care was both feasible and safe.
Introduction
Point-of-care Magnetic Resonance (MR) scanning is a novel and potentially transformative technology, utilising Ultra-Low Field (ULF) permanent magnets to facilitate bedside neuroimaging (1). A range of clinical applications have been proposed in the adult hospital population (2-4), however, image acquisition sequences for neonates require further optimisation. Neonates demonstrate significant maturational differences in tissue properties and typically necessitate markedly different sequence parameters compared to those used for adult subjects (5). There is significant scope for the advancement of neonatal care and disease understanding through the possibility of longitudinal cot-side scanning, as well as scanning the sickest infants, for whom transfer to an MR department outside of the intensive care unit environment is associated with risk of clinical deterioration. Additionally, ULF MRI may benefit neonates in low- and middle-income countries, where the substantial infrastructure and resources required to support traditional high-field MR systems are significant barriers to MRI access.T2 weighted imaging is the mainstay of perinatal brain MRI. We present our findings of iterative T2 weighted neonatal sequence optimisation utilising the 64mT Hyperfine Swoop® portable MRI system.
Methods
Healthy and clinically referred neonates were recruited from St. Thomas’/Evelina Children’s Hospital as part of two NHS UK REC approved studies (12/LO/1247, 19/LO/1384). Infants referred for clinical scans receive Chloral Hydrate sedation as standard, healthy control participants are scanned in natural sleep. All medical support requirements, such as ventilation, intra-venous infusions or thermoregulation are continued throughout scanning.Paired MR brain scans were acquired using the 64 mT Hyperfine MR scanner and a reference standard Philips Achieva 3T MR scanner for all infants. Axial T1w MPRAGE, as well as axial and sagittal T2 w turbo spin echo sequences were acquired on the 3T scanner using neonatal optimised acquisition sequences (6).
To optimise tissue contrast and signal to noise ratio, for age-/pathology-specific changes, 64 mT sequence parameters were iteratively changed to study the impact of resolution, bandwidth, field of view, echo time and inversion time.
Results
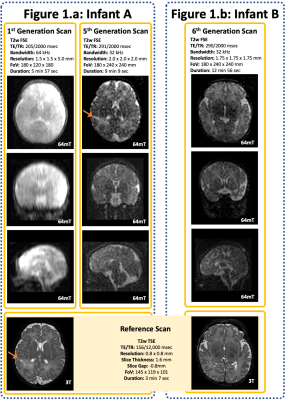
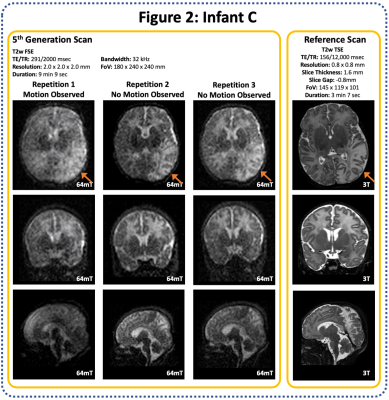
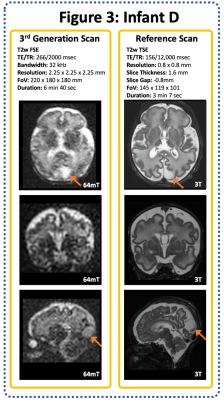
20 infants were scanned (12 clinical referrals, 8 healthy controls). Corrected Gestational Age Range: 34+2 to 43+5 weeks (Median 40+2), age at scan ranged from 1 to 94 days (median 9 days). All scans were well tolerated with no adverse events.A range of pathologies were identified on 64mT and corroborated on reference 3T scans, including focal cortical infarction (Figure 1), widespread white matter and cortical infarction (Figure 2), and CSF cyst (Figure 3).
Switching to an enhanced isotropic resolution and increasing the TE improved tissue contrast on T2 weighted images, revealing cortical grey matter, deep grey matter, and white matter (Figure 1) as well as significantly improved coronal and sagittal orthogonal views. A comparison of our 1st generation (Hyperfine standard T2w Sequence for adults) and 5th generation iterations (bespoke neonatal sequence) is shown in Figure 1; the right superior parietal cortical infarction is identified on the bespoke neonatal sequence, but not on the 1st generation scan.
A reduced signal to noise ratio was observed on our 3rd generation scan (Figure 3), following switching to isotropic resolution but prior to increasing the field of view to increase k-space sampling.
With enhanced resolution and sampling, scan duration increased; 1st Generation T2w scan: 5 min 57 sec, 6th generation scan: 12 min 56 sec. Longer acquisitions are prone to significant motion artifact degradation (Figure 2).
Discussion
We describe the first results and thus demonstrate the feasibility of using ULF portable 64mT MR scanning in neonates. This pilot data demonstrates that bespoke T2 weighted neonatal MR sequences can provide sufficient contrast for tissue differentiation and identify pathological lesions. Scanning with ongoing intensive care was both feasible and safe.Optimised sequences are of significant duration and are thus more prone to motion artefact. Our optimisation continues with further work required on signal averaging, and motion correction. More extensive data collection is required for further validation and to provide an understanding of rate of false-negative and false-positive neonatal scan reporting at 64mT.
Acknowledgements
We thank Dr Nathan Artz for his input during the initial phase of this project.
This work was supported by the Medical Research Council UK Translation Support Award (Grant MR/V036874/1), and The Bill & Melinda Gates Foundation. PC is supported by the Medical Research Council Centre for Neurodevelopmental Disorders, King's College London (Grant MR/N026063/1).
References
1) Deoni, S., et al. Accessible pediatric neuroimaging using a low field strength MRI scanner. NeuroImage 2021;238:118273
2) Mazurek, M. et al. Portable, bedside, low-field magnetic resonance imaging for evaluation of intracerebral hemorrhage. Nature Communications 2021;12(1)
3) Sheth, K., et al. Assessment of Brain Injury Using Portable, Low-Field Magnetic Resonance Imaging at the Bedside of Critically Ill Patients. JAMA Neurology, 2021;78(1):41
4) Turpin, J., et al. Portable Magnetic Resonance Imaging for ICU Patients. Critical Care Explorations 2020:2(12):e0306
5) Schneider, J., Evolution of T1 Relaxation, ADC, and Fractional Anisotropy during Early Brain Maturation: A Serial Imaging Study on Preterm Infants. American Journal of Neuroradiology 2015; 37(1):155
6) Hughes, E., et al. A dedicated neonatal brain imaging system. Magnetic Resonance in Medicine 2017:78(2):C1
Figures