0734
Neuroimaging Findings & Neurological Symptoms in COVID-19 Patients with & without Cancer: A Retrospective Multi-Center Observational Study1Icahn School of Medicine at Mount Sinai, New York, NY, United States, 2BioMedical Engineering and Imaging Institute, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 3Neurology, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 4Radiology, Icahn School of Medicine at Mount Sinai, New York, NY, United States
Synopsis
COVID-19 is known to result in neurological manifestations which sometimes correlate with neuroimaging abnormalities. Understanding these abnormalities requires further systematic study. We undertook a retrospective analysis of 60 MRIs from hospitalized COVID-19 patients (20 intubated, 20 non-brain cancer, and 20 non-intubated/non-cancer) with neurological manifestations. Neuroimaging showed non-infarct parenchymal T2/FLAIR signal hyperintensities (26.7%), acute/subacute infarcts (25%), microhemorrhages (21.7%), chronic infarcts (18.3%), chronic macrohemorrhages (10%), and acute macrohemorrhages (6.7%). Common neurological symptoms were confusion/encephalopathy/delirium (70%), generalized weakness (38.3%), and impaired responsiveness/coma (38.3%). Thus, COVID-19 may be associated with neuroimaging abnormalities. Differences in abnormalities were seen based on permutations of cancer and intubation.
Introduction
Coronavirus 2019 (COVID-19) leads to neurological manifestations [1] often with concordant brain magnetic resonance imaging (MRI) findings [2-4]. While studies have described general MRI findings in these patients, few have focused on specific patient populations, including those with cancer, who may be at greater risk for prothrombotic events such as stroke [5-7]. We conducted a retrospective study involving MRIs obtained on hospitalized COVID-19 patients exhibiting neurological manifestations, stratified by: intubated, non-intubated cancer, and non-intubated without cancer. We aimed to report the neurological symptoms and neuroimaging findings in these three subgroups.Methods
The cohort consisted of 20 intubated patients, 20 patients with non-CNS cancer, and 20 non-cancer patients who were non-intubated. They were hospitalized in the Mount Sinai Health System in New York City between March and June 2020 with COVID-19 diagnosed via real-time polymerase chain reaction and underwent brain MRI during their admissions. Clinical and imaging data were acquired from the EMR and analyzed. MRI scans were performed according to a default brain imaging protocol at 1.5 Tesla on a GE Optima MR450 scanner with routine sequences including axial T2, axial T2 FLAIR, axial GRE, sagittal T1, and diffusion weighted imaging with ADC maps. Three fellowship-trained CAQ neuroradiologists with 5, 15, and 22 years of experience and a trained PGY-4 radiology resident reviewed the MRI scans using a randomized blinded multiple paired readers approach. Brain images were examined for abnormal non-infarct parenchymal T2/FLAIR hyperintensities, acute/subacute and chronic infarcts, and macro- and microhemorrhages based on visual qualitative assessment. Diffusion abnormalities were investigated based on apparent diffusion coefficient maps. Discrepancies in neuroradiological findings were subsequently reassessed for forced adjudication based on consensus between the three fellowship-trained neuroradiologists. Descriptive statistics were calculated.Results
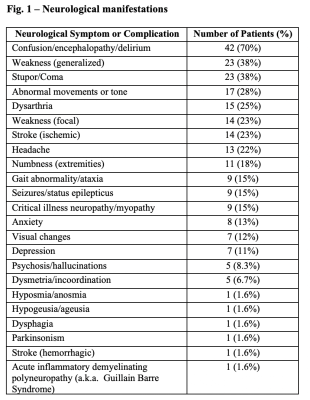
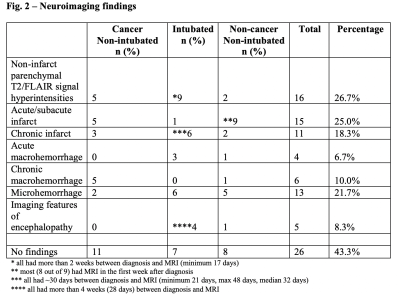
The cohort consistent of 60 patients (34 male, 26 female, 63 ±14 years). MRI scans occurred on average 21 days (±19 days) after diagnosis. Intubated patients received MRI scans later (median 32 days after diagnosis) than non-intubated patients (median 7 days after diagnosis). Most common neurological symptoms/complications included altered mental status (i.e. encephalopathy or delirium) (70%), generalized weakness (38.3%), and impaired responsiveness or coma (38.3%) (Fig. 1). Neuroimaging findings included non-infarct parenchymal T2/FLAIR signal hyperintensities (26.7%), acute/subacute infarcts (25%), microhemorrhages (21.7%), chronic infarcts (18.3%), chronic macrohemorrhages (10%), and acute macrohemorrhages (6.7%). There were no clinically significant qualitative neuroimaging findings in 43.3% of patients, including 11 cancer, 7 intubated, and 8 non-cancer/non-intubated patients (Fig. 2). Hyperintensities were more common in intubated (9/20) than non-intubated cancer (5/20) and non-cancer patients (2/20). Almost all hyperintensities were in patients who had MRI scans more than two weeks after diagnosis. Hyperintensities were further subdivided into two categories: confluent and punctate. Confluent foci were found in 2/20 cancer, 4/20 intubated, and 2/20 non-cancer/non-intubated patients. Punctate foci were found in 3/20 cancer, 5/20 intubated, and 2/20 non-cancer/non-intubated patients. Acute/subacute infarcts were more prevalent in non-cancer/non-intubated (9/20) than non-intubated cancer (5/20) and intubated patients (1/20), while chronic infarcts were more widespread in intubated (6/20) than non-cancer/non-intubated (2/20) and non-intubated cancer patients (3/20). Chronic macrohemorrhages were more common in non-intubated cancer (5/20) than intubated (0/20) and non-cancer/non-intubated patients (1/20). Acute macrohemorrhages were more numerous in intubated (3/20) and non-cancer/non-intubated (1/20) than cancer patients (0/20).Discussion
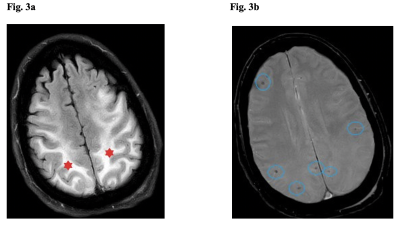
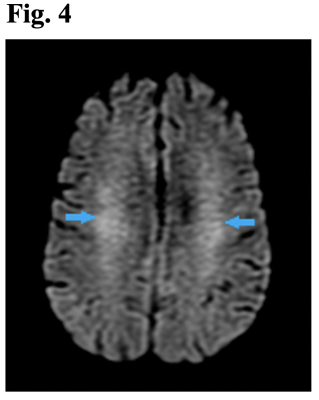
Consistent with prior investigations [8, 9], non-infarct parenchymal T2/FLAIR signal hyperintensities were the most common neuroimaging feature in our COVID-19 patients with neurological manifestations, especially in those intubated. This feature has been attributed to hypoxia, a logical consequence in the low blood oxygen levels afflicting many patients with severe COVID-19 particularly those intubated [10]. In our cohort, the potential relationship with hypoxia applies only to hyperintensities that were confluent foci with diffusion restriction. Acute/subacute and chronic infarcts were also noted, consistent with previous literature [11-13]. While acute/subacute infarcts were more common in non-cancer/non-intubated patients, chronic infarcts were more typical in intubated patients, possibly due to delays in timing of MRI scans in this population and opportunities for more complete disease evolution. In agreement with earlier studies [14-16], microhemorrhages, acute macrohemorrhages, and chronic macrohemorrhages were also detected (Fig. 3). Five out of 6 patients with chronic macrohemorrhages were cancer patients. Cancer patients on chemotherapy may have had pancytopenia, decreasing platelets and increasing bleeding risk, or alternatively blood thinners employed in prothrombotic cancer patients may have introduced a higher risk for macrohemorrhages. Finally, imaging features of encephalopathy, previously documented by others [17], were recorded in several patients, especially among the intubated (4/5) (Fig. 4). The fact that most of these patients were more critically ill, thereby necessitating intubation, makes sense given that encephalopathy is typically seen in sicker patients [18, 19]. Encephalopathy-associated imaging features may also be more apparent in these patients because all had MRI scans over 30 days after diagnosis, providing time for wide-ranging brain damage to develop.Conclusion
Our findings enhance understanding of the imaging findings noted in COVID-19 in patient populations including intubated and cancer patients. Future studies with more patients and prospective designs [20, 21] would be valuable. More granular analysis of brain and brainstem regional volumes using images obtained at higher field strengths is warranted. Finally, diffusion tensor imaging and resting state functional MRI could reveal additional brain regions and networks implicated in COVID-19.Acknowledgements
Acknowledgements:
The patient list was produced from a search for parameters through an informatics database containing COVID-19 clinical data maintained by the Mount Sinai Clinical Intelligence Center (MSCIC). We thank Marco Pereanez, PhD and Valentin Fauveau from MSCIC for performing this search. NJ is the Bludhorn Professor of International Medicine.
Grants/Funding Sources:
- R01CA202911 from NCI (National Cancer Institute)
- R21NS122389 from NINDS (National Institute of Neurological Disorders and Stroke)
Disclosures:
Dr. Balchandani is a named inventor on patents relating to magnetic resonance imaging (MRI) and RF pulse design. The patents have been licensed to GE Healthcare, Siemens AG, and Philips international. NJ receives grant funding paid to her institution for grants unrelated to this work from NINDS (NIH U24NS107201, NIH IU54NS100064), NIH U24NS113849, the American Epilepsy Society and the NORSE Institute. Dr. Navis is a recipient of the NIH Loan Repayment Program for 2020-2022.
Authorship details:
Lily McCarthy and Oleksandr Khegai contributed equally and are the co-first authors. Drs. Nathalie Jette and Priti Balchandani contributed equally and are the co-senior authors.
References
1. Mao, L., et al., Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol, 2020. 77(6): p. 683-690.
2. Ladopoulos, T., et al., COVID-19: Neuroimaging Features of a Pandemic. J Neuroimaging, 2021. 31(2): p. 228-243.
3. Kanne, J.P., et al., COVID-19 Imaging: What We Know Now and What Remains Unknown. Radiology, 2021. 299(3): p. E262-E279.
4. Moonis, G., et al., The Spectrum of Neuroimaging findings on CT and MRI in Adults with Coronavirus Disease (COVID-19). AJR Am J Roentgenol, 2020.
5. Ellul, M.A., et al., Neurological associations of COVID-19. Lancet Neurol, 2020. 19(9): p. 767-783.
6. Pezzini, A. and A. Padovani, Lifting the mask on neurological manifestations of COVID-19. Nat Rev Neurol, 2020. 16(11): p. 636-644.
7. Aaroe, A.E., et al., Potential neurologic and oncologic implications of the novel coronavirus. Neuro Oncol, 2020. 22(7): p. 1050-1051.
8. Nicholson, P., L. Alshafai, and T. Krings, Neuroimaging Findings in Patients with COVID-19. AJNR Am J Neuroradiol, 2020. 41(8): p. 1380-1383.
9. Kremer, S., et al., Neurologic and neuroimaging findings in patients with COVID-19: A retrospective multicenter study. Neurology, 2020. 95(13): p. e1868-e1882.
10. Vogrig, A., et al., Stroke in patients with COVID-19: Clinical and neuroimaging characteristics. Neurosci Lett, 2021. 743: p. 135564.
11. Mahammedi, A., et al., Imaging of Neurologic Disease in Hospitalized Patients with COVID-19: An Italian Multicenter Retrospective Observational Study. Radiology, 2020. 297(2): p. E270-E273.
12. Agarwal, S., et al., Cerebral Microbleeds and Leukoencephalopathy in Critically Ill Patients With COVID-19. Stroke, 2020. 51(9): p. 2649-2655.
13. Mahammedi, A., et al., Brain and Lung Imaging Correlation in Patients with COVID-19: Could the Severity of Lung Disease Reflect the Prevalence of Acute Abnormalities on Neuroimaging? A Global Multicenter Observational Study. AJNR Am J Neuroradiol, 2021.
14. Kremer, S., et al., Brain MRI Findings in Severe COVID-19: A Retrospective Observational Study. Radiology, 2020. 297(2): p. E242-E251.
15. Chen, B., et al., Insights Into Neuroimaging Findings of Patients With Coronavirus Disease 2019 Presenting With Neurological Manifestations. Front Neurol, 2020. 11: p. 593520.
16. Klironomos, S., et al., Nervous System Involvement in Coronavirus Disease 2019: Results from a Retrospective Consecutive Neuroimaging Cohort. Radiology, 2020. 297(3): p. E324-E334.
17. Uginet, M., et al., COVID-19 encephalopathy: Clinical and neurobiological features. J Med Virol, 2021. 93(7): p. 4374-4381.
18. Liotta, E.M., et al., Frequent neurologic manifestations and encephalopathy-associated morbidity in Covid-19 patients. Ann Clin Transl Neurol, 2020. 7(11): p. 2221-2230.
19. Garg, R.K., V.K. Paliwal, and A. Gupta, Encephalopathy in patients with COVID-19: A review. J Med Virol, 2021. 93(1): p. 206-222.
20. Lu, Y., et al., Cerebral Micro-Structural Changes in COVID-19 Patients - An MRI-based 3-month Follow-up Study. EClinicalMedicine, 2020. 25: p. 100484.
21. Kas, A., et al., The cerebral network of COVID-19-related encephalopathy: a longitudinal voxel-based 18F-FDG-PET study. Eur J Nucl Med Mol Imaging, 2021.
Figures