0730
Myocarditis associated with mRNA COVID-19 vaccines: MRI findings
Jitka Starekova1, David A Bluemke1,2, William S Bradham1,3, Thomas M Grist1,2,4, Mark L Schiebler1, and Scott B Reeder1,2,3,4,5
1Radiology, University of Wisconsin-Madison, Madison, WI, United States, 2Medical Physics, University of Wisconsin-Madison, Madison, WI, United States, 3Medicine, University of Wisconsin-Madison, Madison, WI, United States, 4Biomedical Engineering, University of Wisconsin-Madison, Madison, WI, United States, 5Emergency Medicine, University of Wisconsin-Madison, Madison, WI, United States
1Radiology, University of Wisconsin-Madison, Madison, WI, United States, 2Medical Physics, University of Wisconsin-Madison, Madison, WI, United States, 3Medicine, University of Wisconsin-Madison, Madison, WI, United States, 4Biomedical Engineering, University of Wisconsin-Madison, Madison, WI, United States, 5Emergency Medicine, University of Wisconsin-Madison, Madison, WI, United States
Synopsis
Mass immunization campaigns continue in attempts to contain the ongoing COVID-19 pandemic. COVID-19 vaccines authorized for use in the United States include BNT162b2 (Pfizer-BioNTech), mRNA-1273 (Moderna), and JNJ-78436735 (Johnson & Johnson).
To date multiple cases of vaccine-associated inflammation of the heart have been reported. In this work, we describe cardiac MRI findings in 9 patients (adults and children) with clinical and imaging-based diagnosis of myocarditis and/or pericarditis diagnosed shortly after COVID-19 mRNA vaccination at our institution.
INTRODUCTION
Mass immunization campaigns continue in attempts to contain the ongoing COVID-19 pandemic. COVID-19 vaccines currently authorized for use in the United States include BNT162b2 (Pfizer-BioNTech), mRNA-1273 (Moderna), and JNJ-78436735 (Johnson & Johnson).Although preapproval trials of mRNA-based vaccines showed excellent safety profiles1, rare cases of myocarditis with a possible link to mRNA vaccines against COVID-19 have been reported.2,3
In this work, we describe the cardiac MRI findings in patients diagnosed with myocarditis and pericarditis diagnosed shortly after COVID-19 mRNA vaccine administration.
METHODS
In this retrospective IRB approved and HIPAA compliant study, cardiac MRI examinations performed at our institution between January 1 and November 6, 2021, were reviewed for MRI findings of myocarditis and pericarditis. Subsequently, electronic health records (EHR) were reviewed, and patients who received COVID-19 vaccine preceding MRI were included (consecutive sample). Informed consent was waived per IRB protocol. Patients with a history of prior COVID-19 infection were excluded.Cardiac MRI was performed at 1.5 T or 3.0 T (Signa Artist, Signa Premier, Optima MR450w GE Healthcare, Waukesha, WI) including T2-weighted (T2w), cine and late-gadolinium enhancement (LGE) imaging.4 For LGE imaging, 0.2 mmol/kg of gadoterate meglumine (Dotarem®, Guerbet, Roissy, France) intravenous was used, phase-sensitive inversion recovery (PSIR) T1-weighted images were acquired 10-12 minutes after contrast administration. Updated Lake Louise criteria were used for the interpretation of myocardial inflammation.5
Clinical reports were reviewed by 3 cardiovascular radiologists (7–27 years of experience) in consensus. Demographic and clinical data including details of COVID-19 vaccination, 12-lead electrocardiogram (ECG) findings, and serum markers of cardiac injury were documented.
Statistical analysis was performed using Excel (v16.30, Microsoft, Washington).
RESULTS
9 patients (7:2 male:female; age range, 13–38 years, median 21 years) were identified who had abnormal MRI findings and were vaccinated against COVID-19 preceding MRI. All patients experienced acute onset of cardiac symptoms including chest pain in a short temporal window after receiving mRNA COVID-19 vaccines (2 after mRNA-1273 2nd dose, 6 after BNT162b2 2nd dose, and 1 after BNT162b2 3rd dose). All patients were hospitalized with a diagnosis of acute myocarditis and/or pericarditis, with 7/9 receiving cardiac MRI during their hospital stay and 2/9 first during the clinical follow-up approximately 3 months later (Table 1). Electrocardiogram findings were abnormal in all but one patients and cardiac troponin levels were abnormal in all patients (Table 1).Patients 1-6 received their second dose of BNT162b2 vaccine one to three days (range) before, and Patient 7 received the booster shot of BNT162b2 vaccine 5 days before onset of cardiac symptoms. Patients 8 and 9 both received their second dose of mRNA-1273 3 days before onset of chest pain.
In all patients, MRI showed findings of myocarditis and/or pericarditis, including nonischemic pattern of late gadolinium enhancement, corresponding signal abnormalities on T2w images, and pericardial enhancement (Figure 1, Table 1). In Patients 5 and 6 inflammatory changes were mild, as expected after longer follow-up (Table 1). After initial diagnosis of vaccine-associated myocarditis, follow-up MRI exams in Patients 1 and Patient 2 performed 136 and 182 days after the initial cardiac MRI showed a decrease in inflammatory change most evident on T2 weighed images (Figure 1).
COVID-19 testing at the time of diagnosis and/or history of prior COVID-19 was negative in all cases. No respiratory symptoms, prodrome, or skin rash were present prior to vaccination. Furthermore, medical history did not reveal any preexisting cardiac disease in these patients.
DISCUSSION
In this study, we reported the MRI findings of myocarditis and/or pericarditis associated with mRNA COVID-19 vaccination in 9 patients (8 patients after 2nd vaccine, 1 patient after booster shot mRNA vaccine). The clinical presentation (acute chest pain, abnormal troponin level, electrocardiogram finding), vaccination-symptoms timing, and negative COVID-19 history raised concern for vaccine-related myocarditis.Vaccine-related myocarditis is rare compared with typical viral myocarditis6, and the associations we observed do not indicate causation. However, the reported cases illustrate cardiac MRI findings that may be encountered by the imaging physician.
To date, 2,907 cases of myo-/pericarditis following COVID-19 vaccination have been reported in the Centers for Disease Control and Prevention’s Vaccine Adverse Event Reporting System, or VAERS, database7. Because VAERS requires active reporting, there is a risk of bias, and the confidence in prior reports is therefore unclear. Our observations are consistent with other case series of hospitalized patients, showing that most patients with associated myocarditis are younger men presenting after the second dose of mRNA COVID-19 vaccine.3,8
Based on emerging data, pharmacovigilance for myocardial injury related to mRNA-based vaccination should be encouraged during the ongoing vaccination program in adults and children including the booster vaccination shots.
In conclusion, vaccine-related cardiac injury should be considered in the differential diagnosis in patients, adults and children, recently vaccinated against COVID-19 who present with acute cardiac symptoms.
Acknowledgements
The authors wish to acknowledge support from GE Healthcare and Bracco Diagnostic who provide research support to the University of Wisconsin. Dr. Reeder is a Romnes Faculty Fellow, and has received an award granted by the University of Wisconsin-Madison Office of the Vice Chancellor for Research and Graduate Education with funding from the Wisconsin Alumni Research Foundation.References
- Dagan N, Barda N, Balicer RD. Adverse Effects after BNT162b2 Vaccine and SARS-CoV-2 Infection, According to Age and Sex. N Engl J Med. Published online October 27, 2021:NEJMc2115045. doi:10.1056/NEJMc21150452.
- Starekova J, Bluemke DA, Bradham WS, Grist TM, Schiebler ML, Reeder SB. Myocarditis Associated with mRNA COVID-19 Vaccination. Radiology. 2021 Nov;301(2):E409-E411. doi: 10.1148/radiol.2021211430. Epub 2021 Jul 20.
- Montgomery J, Ryan M, Engler R, et al. Myocarditis Following Immunization With mRNA COVID-19 Vaccines in Members of the US Military. JAMA Cardiol. Published online June 29, 2021. doi:10.1001/jamacardio.2021.28334.
- Starekova J, Bluemke DA, Bradham WS, et al. Evaluation for Myocarditis in Competitive Student Athletes Recovering From Coronavirus Disease 2019 With Cardiac Magnetic Resonance Imaging. JAMA Cardiol. Published online January 14, 2021. doi:10.1001/jamacardio.2020.74445.
- Ferreira VM, Schulz-Menger J, Holmvang G, et al. Cardiovascular Magnetic Resonance in Nonischemic Myocardial Inflammation. Journal of the American College of Cardiology. 2018;72(24):3158-3176. doi:10.1016/j.jacc.2018.09.0727.
- Mei R, Raschi E, Forcesi E, Diemberger I, De Ponti F, Poluzzi E. Myocarditis and pericarditis after immunization: Gaining insights through the Vaccine Adverse Event Reporting System. Int J Cardiol 2018;273:183–186.8.
- United States Department of Health and Human Services (DHHS), Pub- lic Health Service (PHS), Centers for Disease Control (CDC)/Food and Drug Administration (FDA), Vaccine Adverse Event Reporting System (VAERS) 1990 - 10/29/2021, CDC WONDER On-line Database. http:// wonder.cdc.gov/vaers.html. Accessed Nov 6, 2021.9.
- Dionne A, Sperotto F, Chamberlain S, et al. Association of Myocarditis With BNT162b2 Messenger RNA COVID-19 Vaccine in a Case Series of Children. JAMA Cardiol. 2021. 2021 Aug 10:e213471. doi: 10.1001/jamacardio.2021.3471. Epub ahead of print.
Figures
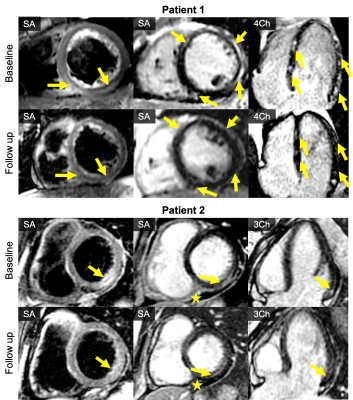
Figure 1: Cardiac MRI of 2 patients with acute myocarditis (baseline MRI) performed in Patient 1 (21-year-old male) 3 days, and in Patient 2 (32-year-old female) 4 days after vaccination with BNT162b2. Follow up MRI of both patients performed 4 months (Patient 1) and 5 months (Patient 2) after the initial cardiac MRI showed decrease in edema (T2 DIR-FSE images, left column) and late gadolinium enhancement (T1 PSIR images, middle and right column), depicted by yellow arrows. Small pericardial effusion in Patient 2 with subtle pericardial enhancement (star) remained nearly unchanged.
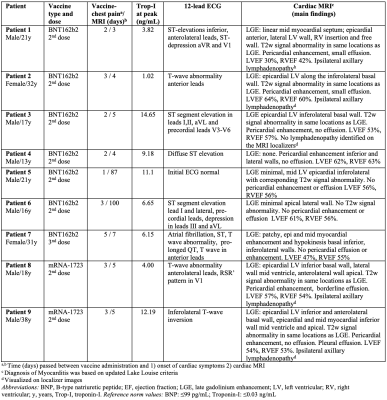
Table 1: Results in 9 patients diagnosed with myocarditis or pericarditis in short temporal relation to mRNA COVID-19 vaccine. All patients were hospitalized, and had negative history of prior COVID-19.
DOI: https://doi.org/10.58530/2022/0730