0723
Development of MRI-based 3D Radiomics Signatures for Preoperative Risk Stratification of Patients with Histology-proven Endometrial Cancer1Medical Physics Unit, McGill University, Montreal, QC, Canada, 2Cancer Research UK Cambridge Institute, University of Cambridge, Cambridge, United Kingdom, 3Department of Diagnostic Radiology, McGill University, Montreal, QC, Canada, 4Department of Radiology, Kobe University Graduate School of Medicine, Kobe, Japan, 5Department of Radiology, Cochin Hospital, AP-HP.Centre, Paris, France, 6Faculté de Médecine, Université de Paris, Paris, France, 7Department of Precision Medicine, GROW - School for Oncology and Developmental Biology, Maastricht University, Maastricht, Netherlands, 8Department of Computer Science, Université de Sherbrooke, Sherbrooke, QC, Canada, 9Augmented Intelligence & Precision Health Laboratory, Research Institute of McGill University Health Centre, Montreal, QC, Canada, 10Research Institute of McGill University Health Centre, Montreal, QC, Canada, 11Department of Obstetrics and Gynecology, McGill University Health Centre, Montreal, QC, Canada, 12School of Computer Science, McGill University, Montreal, QC, Canada
Synopsis
Radiomics analysis on standard MRI prior to surgery holds potential to help identifying high-risk histopathological features of endometrial carcinoma, including FIGO stage, deep myometrial invasion, lymphovascular space invasion and tumor grade, thus supporting preoperative risk stratification for optimal patient management. This dual-center retrospective study evaluated the role of radiomics to assess high-risk phenotypes of endometrial cancer in women who underwent 1.5-T MRI before hysterectomy. Radiomics-based machine learning models provided consistent clinically acceptable performance for differentiating early from advanced FIGO stage endometrial carcinoma and for differentiating low- from high-risk histopathological markers in two independent datasets from different institutions on preoperative MRI.
Introduction
High-risk histopathological features including FIGO stage, deep myometrial invasion (MI), lymphovascular invasion (LVSI), and high tumor grade are widely used to stratify endometrial carcinoma patients for therapy. MRI has taken an essential role in enabling the identification of those phenotypes that can be visualized and staged on imaging1. Radiomics analysis on standard MRI prior to surgery holds potential for supporting preoperative risk stratification and optimal patient management.Thus, we aimed to construct and validate random forest (RF) classifiers of multiparametric MRI (mpMRI)-based 3D radiomics features to predict in a robust fashion advanced FIGO stage (≥IB), presence of deep MI, LVSI and high-grade (grade 3 endometrioid carcinoma or non-endometrioid histologies) endometrial carcinomas, and to compare our findings to preoperative consensus readings from radiologists as well as the final histopathological findings at hysterectomy.
Methods
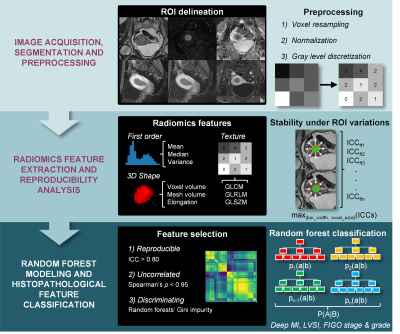
This dual-center institutional review board-approved retrospective study included endometrial cancer patients who underwent one MRI scan before hysterectomy between January 2011 and July 2015. All MRI examinations were performed on 1.5-T MRI systems (Institution 1: Signa Excite; GE Healthcare; Institution 2: Magnetom AvantoVB, Siemens Healthineers) using a vendor-provided pelvic phased-array surface coil. The MRI examinations were performed in the supine position and included standard diagnostic sequences: fast spin echo T2-weighted imaging (T2WI), echo planar imaging diffusion-weighted imaging (DWI, b = 0 and 1000 s/mm2), and three-dimensional gradient-echo T1-weighted dynamic contrast-enhanced (DCE)-MRI. Apparent diffusion coefficient (ADC) maps were derived from DWI acquisitions. Patients were administered 40mg of hyoscine butylbromide intramuscularly or 1mg glucagon intravenously before image acquisition, to reduce bowel and uterine peristalsis. DCE-MRI of the pelvis was performed after administration of 0.1mmol/kg of body weight of gadolinium chelate. Images were acquired during multiple phases following intravenous administration of gadolinium chelate in either sagittal or axial oblique planes (pre-contrast, post-contrast at 25, 60, and 120 seconds in the sagittal plane, and post-contrast at 240 seconds in the axial oblique plane).Preoperative 3D radiomics features were extracted from images of all MR sequences after manual segmentation of the tumor (Figure 1). Briefly, radiomics features were computed using the Image Biomarker Standardization Initiative (IBSI)-compliant2 Pyradiomics software package3. Feature extraction reproducibility was then assessed. Features that were robust to changes in image preprocessing parameters and variations in contour delineation (intra-class correlation>0.8) which were uncorrelated with each other (Spearman’s rho<0.95) were retained for subsequent analysis. Based on those retained features, an initial random forest (RF) classifier was trained in order to determine the predictive power of individual features. The top 5 features were retained, a final RF classifier based on these 5 features was trained, and then applied to the independent testing dataset. Receiver operating characteristic (ROC) analysis was performed and the areas under the ROC curves (AUC) were reported together with diagnostic performance metrics.
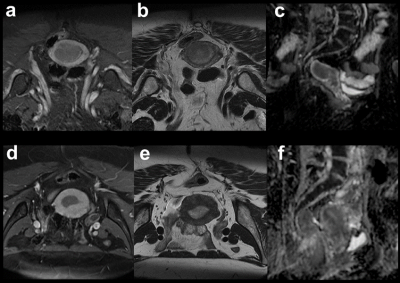
In the population recruited at the first institution, FIGO staging on the mpMRI examinations was performed by five board-certified radiologists during routine clinical assessment with at least 5 years of experience in gyne-oncology MRI interpretation. All MRI examinations were reviewed in an interdisciplinary clinical board prior to surgery for quality assurance and final MRI staging was assigned by consensus. Representative cases are reported in Figure 2. Diagnostic performance measures for this final diagnosis and 95% confidence intervals (CI) were computed and McNemar tests were performed to compare the binary predictions of radiomics models and consensus radiologists’ readings.
Results
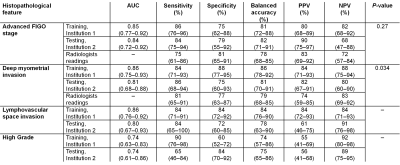
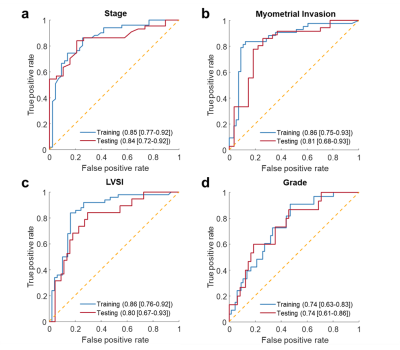
A total of 157 patients were included, 94 at the first institution (mean age, 65.5±10.6 years; range: 43-90) and 63 at the second institution (age, 67.1±11.5 years; 44-88). The best combination of radiomics features for dichotomizing FIGO stage, deep MI, LVSI, and tumor grade reported in Figure 3 led to AUCs of and 0.84(95% CI, 0.72-0.92), 0.81(0.68-0.88), 0.80(0.67-0.93), and 0.74(0.61-0.86) on the testing dataset, respectively (Figure 4 and Figure 5).In the population recruited at the first institution, the radiomics models provided non-inferior performance compared to consensus readings for identifying advanced FIGO stage (P=0.27) and superior performance for identifying deep MI (P=0.034).
Discussion
The reported performance of radiomics modeling for differentiating early from advanced FIGO stage was comparable with that of consensus radiologists’ readings on the training dataset. The dedicated radiomics model for identifying deep MI outperformed radiologists in preoperative patient stratification in the training population. Considering the current radiologists’ workload, having preoperative MRI-derived radiomics risk stratification with similar to higher diagnostic performance when compared to consensus readings of experienced radiologists may provide junior radiologists with enhanced decision making capabilities while alleviating the pressure on radiology departments. Moreover, the European Society of Medical Oncology (ESMO) considers FIGO IA as the only low risk stage4, thus showing the potential of our MRI-based radiomics signature for endometrial carcinoma risk stratification prior to therapy.Conclusion
In conclusion, our radiomics-based RF modeling provided consistent clinically acceptable performance for differentiating early from advanced FIGO stage endometrial carcinoma as well as differentiating low- from high-risk histological markers (deep MI, LVSI, and high-grade) in two independent datasets from different institutions on preoperative mpMRI. Accurate noninvasive preoperative identification of patients with low risk endometrial carcinoma from machine learning-derived radiomics features may decrease surgical time by obviating the need for sentinel lymph node dissection and the potential attendant lymphadenectomy-related complications.Acknowledgements
This work was jointly funded by the Fonds de recherche du Québec - Santé (FRQS) and the Fondation de l'association des radiologistes du Québec (FARQ). TLL would like to acknowledge financial support by the NSERC Alexander Graham Bell Canada Graduate Scholarship. MV acknowledges funding from the Canada CIFAR AI Chairs Program.References
1. Freeman SJ, Aly AM, Kataoka MY, Addley HC, Reinhold C, Sala E. The revised FIGO staging system for uterine malignancies: implications for MR imaging. Radiographics 2012;32(6):1805-1827. doi: 10.1148/rg.3261255192. Zwanenburg A, Vallieres M, Abdalah MA, Aerts H, Andrearczyk V, Apte A, Ashrafinia S, Bakas S, Beukinga RJ, Boellaard R, Bogowicz M, Boldrini L, Buvat I, Cook GJR, Davatzikos C, Depeursinge A, Desseroit MC, Dinapoli N, Dinh CV, Echegaray S, El Naqa I, Fedorov AY, Gatta R, Gillies RJ, Goh V, Gotz M, Guckenberger M, Ha SM, Hatt M, Isensee F, Lambin P, Leger S, Leijenaar RTH, Lenkowicz J, Lippert F, Losnegard A, Maier-Hein KH, Morin O, Muller H, Napel S, Nioche C, Orlhac F, Pati S, Pfaehler EAG, Rahmim A, Rao AUK, Scherer J, Siddique MM, Sijtsema NM, Socarras Fernandez J, Spezi E, Steenbakkers R, Tanadini-Lang S, Thorwarth D, Troost EGC, Upadhaya T, Valentini V, van Dijk LV, van Griethuysen J, van Velden FHP, Whybra P, Richter C, Lock S. The Image Biomarker Standardization Initiative: Standardized Quantitative Radiomics for High-Throughput Image-based Phenotyping. Radiology 2020;295(2):328-338. doi: 10.1148/radiol.2020191145
3. van Griethuysen JJM, Fedorov A, Parmar C, Hosny A, Aucoin N, Narayan V, Beets-Tan RGH, Fillion-Robin JC, Pieper S, Aerts H. Computational Radiomics System to Decode the Radiographic Phenotype. Cancer Res 2017;77(21):e104-e107. doi: 10.1158/0008-5472.CAN-17-0339
4. Colombo N, Preti E, Landoni F, Carinelli S, Colombo A, Marini C, Sessa C, Group EGW. Endometrial cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24 Suppl 6:vi33-38. doi: 10.1093/annonc/mdt353
Figures