0720
Blood-brain barrier water exchange rate is associated with cognitive performance in mild cognitive impairment and early Alzheimer’s disease1School of Psychology and Centre for Brain Research, University of Auckland, Auckland, New Zealand, 2Brain Research New Zealand - Rangahau Roro Aotearoa, Centre of Research Excellence, New Zealand, Auckland, New Zealand, 3Centre for Advanced MRI, University of Auckland, Auckland, New Zealand, 4Keck School of Medicine, University of Southern California, Los Angeles, CA, United States, 5Auckland Bioengineering Institute, University of Auckland, Auckland, New Zealand, 6Department of Engineering Science, University of Auckland, Auckland, New Zealand, 7Department of Pharmacology and Centre for Brain Research, University of Auckland, Auckland, New Zealand, 8Department of Brain Repair and Rehabilitation, UCL Queen Square Institute of Neurology, University College London, London, United Kingdom, 9Dementia Research Centre, UCL Queen Square Institute of Neurology, University College London, London, United Kingdom
Synopsis
Blood-brain barrier (BBB) dysfunction has been observed in multiple neurodegenerative conditions, including mild cognitive impairment (MCI) and Alzheimer’s disease (AD). However recent concerns on the repeated use of Gadolinium based contrast agents (GBCAs), prompted us to investigate alternative, non-invasive methods for measuring BBB health. Diffusion-prepared arterial spin labelling (DP-ASL) imaging was implemented at 3T to determine water exchange rates (Kw) in 55 participants, comprising MCI, early AD and control participants. We found Kw to be associated with cognitive performance, suggesting it may be a useful imaging biomarker of early AD pathology.
INTRODUCTION
Blood-brain barrier (BBB) breakdown is increased in Alzheimer’s disease (AD) and is associated with neuropathology [1]. Subtle increases in BBB permeability have been reported in mild cognitive impairment (MCI) and Alzheimer’s disease (AD) with MRI using gadolinium based contrast agents [2], [3]. Measuring water exchange across the BBB using Arterial Spin Labelling (ASL) MRI may be an alternative to contrast-enhanced MRI to detect changes in barrier permeability [4]. Because of the small size of water molecules relative to gadolinium chelates, ASL may have greater sensitivity to the paracellular ‘leakiness’ of the BBB. Diffusion-prepared (DP) ASL [5], [6] and Multi-echo (ME) ASL [7], [8] are two alternative methods that have been proposed to investigate BBB permeability, collectively BBB-ASL. In this work we present DP-ASL results measuring water exchange rates in MCI and AD and age-matched cognitively healthy participants.METHODS
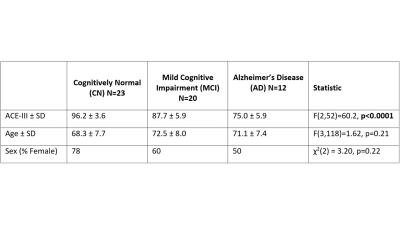
Images were acquired using a MAGNETOM Skyra 3T MR scanner (Siemens Healthcare, Erlangen, Germany) and 32-channel head coil. DP-ASL imaging was performed as described previously [6] to estimate water exchange rate (Kw), cerebral blood flow (CBF) and arterial transit time (ATT) in a single scan. Four sets of data were collected with pCASL labelling: two with post-labelling delay (PLD) = 900ms, and b-values of 0 and 14s/mm2 repeated 11 times each to estimate ATT [9]; two data sets with PLD = 1800 ms and b-values: 0 and 50 s/mm2 repeated 15 times each to separate tissue and microvascular labelled signal and estimate water exchange rate (Kw). A single non-labelled image collected at a delay of 2000ms was used to calibrate perfusion weighted images (generated from the PLD = 1800 ms and b = 0 s/mm2 ) and estimate CBF in ml/min/100g [10]. Scan parameters common to all DP-ASL data were TR = 4200ms, TE = 36.9 ms, FOV = 224 mm, matrix size = 64×64, 12 slices (10% oversampling), resolution = 3.5×3.5×8mm3, labelling duration = 1500 ms, using a 3D GRASE readout, yielding a total scan time of 7’29’’. Sixty-five people enrolled in the Dementia Prevention Research Clinic participated in the study (recruitment is ongoing). All participants were classified following a multi-disciplinary assessment and extensive neuropsychological testing including the Addenbrooke's Cognitive Examination-III (ACE-III). Data from ten participants were excluded from the analysis due to low labelling efficiency. Of the remaining 55 participants, the number classified into each cognitive status group and their demographics are provided as tabulated data in Figure 1. Kw, CBF and ATT maps were determined voxel-wise as previously described [6]. Native space maps were transformed into a common standard space template (Montreal Neurological Institute (MNI)) and mean values extracted from the whole brain, and large regions relevant to AD, the temporal and parietal lobe. Age and ACE-III were compared across the 3 groups using ANOVAs, and a χ2 test was used to compare frequency distribution of sex across the groups. Correlations between Kw, CBF, ATT and ACE-III across all groups were calculated with Pearson’s correlation coefficient.RESULTS
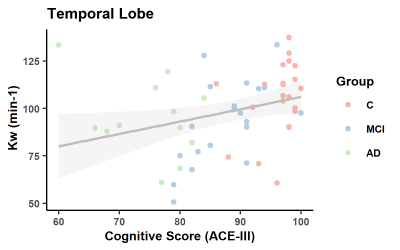
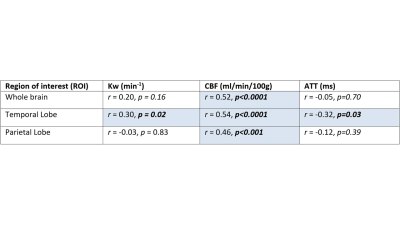
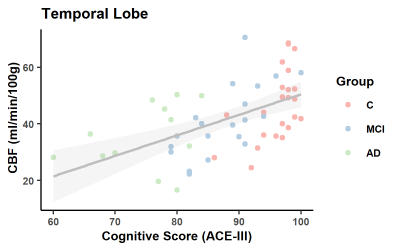
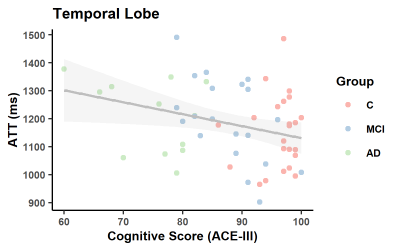
As expected, ACE-III, was statistically different across the groups and in all subsequent pair-wise comparisons (Figure 1). No significant between-group differences were found for age and sex. In the control group, estimates of whole brain Kw (100±18/min), CBF (42±9 ml/100g/min) and ATT (1205±138ms) are comparable to those previously reported [11].Figure 2 shows a positive correlation between Kw in the temporal lobe and ACE-III, i.e. lower water exchange rates correlate with reduced cognitive abilities. Correlations of Kw in the whole brain and parietal lobe were not significant (see tabulated data in Figure 3 for all correlation results). CBF was strongly correlated with ACE-III in all 3 ROIs (see Figure 4 for temporal lobe CBF data plotted). As indicated by the data in Figure 5, a negative correlation was found between ATT and ACE-III in the temporal lobe, suggesting longer transit times in this region in MCI and AD [12], [13].
DISCUSSION AND CONCLUSIONS
In this work we used DP-ASL to measure water exchange rate, Kw as a non-contrast method for examining BBB dysfunction. Values for Kw in the older controls were comparable to those previously reported [11]. Our results measuring Kw in MCI and AD participants suggest an overall decrease in water exchange rates across the BBB in the cognitively impaired, counter to our original hypothesis of increased Kw in the presence of a “leaky” BBB in MCI and AD [2], [14]. Measuring water transport with ASL is sensitive to both paracellular water transport (potentially increasing Kw with tight-junction break down) and transcellular water transport, via aquaporin 4 (AQP4) channels [4] (potentially decreasing Kw with reduced AQP4 water transport). Reduced AQP4 perivascular polarisation has been found in the AD brain [15] and in animal models leads to reduced clearance of amyloid-β [16]. Further, in cognitively normal subjects, lower water exchange rates were correlated with reduced CSF amyloid-β clearance [11]. These results combined suggest that BBB-ASL could be sensitive to an alternative mechanism, such as amyloid clearance function, in MCI and AD compared to gadolinium contrast agent approaches. Measurement of cerebral amyloid load, using 18F-florbetaben positron emission tomography to investigate the relationship of cerebral pathology with CBF and Kw in the same subjects as those presented here is underway.Acknowledgements
This work is funded by Brain Research New Zealand - Rangahau Roro Aotearoa, Centre of Research Excellence, New Zealand. CM was partly funded by the Sir Thomas and Lady Duncan Trust, and the Heath Research Council of New Zealand. We would like to acknowledge all the staff at Dementia Prevention Research Clinic in Auckland. LT and MD contributed equally to this work.References
[1] P. J. Narayan et al., “Assessing fibrinogen extravasation into Alzheimer’s disease brain using high-content screening of brain tissue microarrays,” Journal of Neuroscience Methods, vol. 247, pp. 41–49, May 2015, doi: 10.1016/j.jneumeth.2015.03.017.
[2] H. J. van de Haar et al., “Blood-Brain Barrier Leakage in Patients with Early Alzheimer Disease,” Radiology, vol. 281, no. 2, pp. 527–535, Nov. 2016, doi: 10.1148/radiol.2016152244.
[3] D. A. Nation et al., “Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction,” Nat Med, vol. 25, no. 2, pp. 270–276, Feb. 2019, doi: 10.1038/s41591-018-0297-y.
[4] B. R. Dickie, G. J. M. Parker, and L. M. Parkes, “Measuring water exchange across the blood-brain barrier using MRI,” Progress in Nuclear Magnetic Resonance Spectroscopy, Sep. 2019, doi: 10.1016/j.pnmrs.2019.09.002.
[5] K. s. St. Lawrence, D. Owen, and D. J. J. Wang, “A two-stage approach for measuring vascular water exchange and arterial transit time by diffusion-weighted perfusion MRI,” Magnetic Resonance in Medicine, vol. 67, no. 5, pp. 1275–1284, 2012, doi: 10.1002/mrm.23104.
[6] X. Shao, S. J. Ma, M. Casey, L. D’Orazio, J. M. Ringman, and D. J. J. Wang, “Mapping water exchange across the blood–brain barrier using 3D diffusion-prepared arterial spin labeled perfusion MRI,” Magnetic Resonance in Medicine, vol. 81, no. 5, pp. 3065–3079, 2019, doi: 10.1002/mrm.27632.
[7] Gregori Johannes, Schuff Norbert, Kern Rolf, and Günther Matthias, “T2‐based arterial spin labeling measurements of blood to tissue water transfer in human brain,” Journal of Magnetic Resonance Imaging, vol. 37, no. 2, pp. 332–342, Sep. 2012, doi: 10.1002/jmri.23822.
[8] Y. Ohene et al., “Non-invasive MRI of brain clearance pathways using multiple echo time arterial spin labelling: an aquaporin-4 study,” NeuroImage, vol. 188, pp. 515–523, Mar. 2019, doi: 10.1016/j.neuroimage.2018.12.026.
[9] J. Wang et al., “Arterial transit time imaging with flow encoding arterial spin tagging (FEAST),” Magn. Reson. Med., vol. 50, no. 3, pp. 599–607, Sep. 2003, doi: 10.1002/mrm.10559.
[10] D. C. Alsop et al., “Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia,” Magn. Reson. Med., vol. 73, no. 1, pp. 102–116, Jan. 2015, doi: 10.1002/mrm.25197.
[11] B. T. Gold et al., “Water exchange rate across the blood-brain barrier is associated with CSF amyloid-β 42 in healthy older adults,” Alzheimers Dement, May 2021, doi: 10.1002/alz.12357.
[12] Z. Shirzadi, B. Stefanovic, H. J. M. M. Mutsaerts, M. Masellis, B. J. MacIntosh, and for the Alzheimer’s Disease Neuroimaging Initiative, “Classifying cognitive impairment based on the spatial heterogeneity of cerebral blood flow images: ASL-CBF Spatial Heterogeneity in AD,” Journal of Magnetic Resonance Imaging, Jan. 2019, doi: 10.1002/jmri.26650.
[13] H. K. F. Mak et al., “Quantitative Assessment of Cerebral Hemodynamic Parameters by QUASAR Arterial Spin Labeling in Alzheimer’s Disease and Cognitively Normal Elderly Adults at 3-Tesla,” Journal of Alzheimer’s Disease, vol. 31, no. 1, pp. 33–44, Jan. 2012, doi: 10.3233/JAD-2012-111877.
[14] M. D. Sweeney, A. P. Sagare, and B. V. Zlokovic, “Blood–brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders,” Nature Reviews Neurology, vol. 14, no. 3, pp. 133–150, Mar. 2018, doi: 10.1038/nrneurol.2017.188.
[15] D. M. Zeppenfeld, M. Simon, and D. Haswell, “Association of Perivascular Localization of Aquaporin-4 With Cognition and Alzheimer Disease in Aging Brains | Dementia and Cognitive Impairment | JAMA Neurology | JAMA Network.” https://jamanetwork.com/journals/jamaneurology/article-abstract/2587489 (accessed Nov. 06, 2019).
[16] J. J. Iliff et al., “A Paravascular Pathway Facilitates CSF Flow Through the Brain Parenchyma and the Clearance of Interstitial Solutes, Including Amyloid β,” Science Translational Medicine, vol. 4, no. 147, pp. 147ra111-147ra111, Aug. 2012, doi: 10.1126/scitranslmed.3003748.Figures