0719
Examining cerebrovascular burden across the cognitive continuum in older adults with and without evidence of amyloidosis1University of Wisconsin, Madison, Madison, WI, United States
Synopsis
In this study we investigated cerebrovascular health and vascular contributions to cognitive impairment in the presence and absence of amyloidosis. We employed various neuroimaging techniques including 4D-Flow, multi-delay ASL, T2-FLAIR, and structural T1 for vascular and structural biomarkers. β-amyloid (Aβ) burden was determined from PET imaging data. Data supports the notion that vascular dysfunction occurs in the presence and absence of Aβ, albeit with differing manifestations leading to cognitive decline.
Introduction:
Emerging evidence suggests that once initiated Aβ accumulation in Alzheimer’s disease follows a remarkably predictable time course1,2, and may be present 20 or more years prior to the dementia stage of the disease3,4. However, the eventual symptom expression is heterogeneous and moderately predicted by current Aβ markers5. Cerebrovascular disease (CVD) is another major risk factor for dementia6 that can independently lead to cognitive decline by ischemic and inflammatory pathways7. Recent studies indicate vascular disease can interact with Aβ pathology6,8,9. In this scenario, altered Aβ waste clearance driven by vascular induction has been hypothesized as a mechanistic pathway (glymphatic flow10, IPAD11). However, vascular and Alzheimer’s disease (AD) pathology interactions and effects on cognition have yet to be fully characterized in human studies. In this work, we characterized cerebrovascular health in human subjects spanning the cognitive spectrum with and without presence of Aβ using different neuroimaging techniques (Figure 1). MRI based vascular markers included macro- and micro- vascular perfusion and vessel stiffness, and white matter hyperintensities (WMHs) from 4D-Flow, multi-delay ASL, and T2-FLAIR. AD biomarkers of Aβ deposition were quantified using 11C-PiB.Methods:
Subjects: A total of 70 subjects are included in this work (Fig2). Subjects were clinically characterized into four groups: cognitively unimpaired (CU), CU-Declining12 (these are subjects who are at-risk of progressing to clinical mild cognitive impairment (MCI) based on traditional neuropsychological data), clinical MCI, and clinical dementia. MRI: Data were acquired on a 3.0T system (MR750, GE Healthcare) using a 32-channel head coil. Vascular imaging: Whole brain 4D-Flow data were acquired with the following imaging parameters Venc=80cm/s, imaging volume=22x22x16cm3, TR/TE=7.7/2.5ms, scantime~5.6min.13 Pseudo-continuous arterial spin labeling (ASL) (1.875x1.875mm2 in plane resolution, 4mm slice thickness) with three post labeling delays (1.0, 1.8, 2.7 sec) was used to provide a measure of tissue perfusion corrected for arrival time (5 min). Structural imaging: 3D T1-weighted and T2-FLAIR images were also collected. Macro- and micro- vessel stiffness, CBF and other parameters: Velocity data were retrospectively reconstructed to the cardiac cycle (20 frames) using GPU accelerated (SigPy)14 iterative SENSE with JSENSE15 sensitivity maps and a local-low rank temporal constraint16. Background phase and velocity aliasing corrections were performed.17 Hemodynamic measurements were extracted from intracranial arteries including the petrous internal carotid arteries (ICAs), basilar artery (BA), middle cerebral arteries (MCAs), and veins (superior sagittal sinus (SSS)). Vessels were segmented automatically in MATLAB (Mathworks, Natick, MA).18 Quantified flow parameters included: total cerebral blood flow, trans-capillary pulse wave delay, and flow pulsatility index (PI). Trans-capillary pulse wave delay was defined as the time shift between arterial and venous cardiac waveforms measured from time-to-upstroke (maximum acceleration).9 Micro-vascular tissue perfusion was extracted from transit time corrected CBF maps using SPM12 and CAT12. Intracranial volumes (ICVs) and hippocampal volume were segmented using T1-weighted data, SPM12,19 and FSL FIRST20. WMHs lesion volumes were segmented from 3D T2-FLAIR images using a lesion segmentation tool for SPM12.21 PET: Aβ burden was assessed using 11C-PiB PET imaging.22 Amyloid positivity (A-, A+) was established as mean cortical PiB DVR >1.16 based on Gaussian mixture modeling from a larger sample.23 For A+ participants, amyloid time (i.e., estimated duration A+) was estimated using the sampled iterative local approximation (SILA) algorithm at the time of the last PiB scan.24 Pairwise statistical differences were assessed using ANOVA followed by post hoc analysis using the Tukey‐Kramer method (P<0.05 significance).Results:
Demographics, AD, and vascular imaging biomarkers are summarized in figures 2 and 3. WMHs volumes and macro-vascular blood flow was similar between A+ groups; however, micro-vascular tissue perfusion was statically higher in A+ CU when compared to other A+ groups in the GM, WM, and hippocampus (Figures 3 and 4). No statistical differences were measured between the A- groups in WMHs volumes, macro-vascular blood flow, and micro-vascular tissue perfusion. Trans-capillary pulse wave delay was statistically longer in A+ CU when compared to A+ CU-Declining, A+ MCI, and A+ dementia (Figure 5 A). However, flow PI in the ICA and MCA was similar between A+ groups except with higher MCA flow PI in A+ dementia when compared to A+ CU (Figure 5 B). For A- groups trans-capillary pulse wave delay was similar; yet, flow PI was statistically higher in A- CU-Declining and A- MCI when compared to A- CU in the ICAs and MCAs (Figure 5 C, D). Flow PI in A+ CU was higher than in A- CU.Discussion and Conclusions:
Decreased micro-vascular tissue perfusion and shorter trans-capillary pulse wave delay in A+ subjects with cognitive alterations (e.g. CU-Declining, MCI, Dementia) compared to A+ CU indicates decreased metabolism and increased small vessel/capillary stiffness may contribute to cognitive decline and/or are induced/accelerated by AD proteinopathy (amyloidosis). In contrast, for A- subjects the lack of differences in micro-vascular tissue perfusion and trans-capillary pulse wave delay but significant differences in arterial flow pulsatility index (ICA, MCA) between A- CU, CU-Declining, and MCI suggests macro-vascular arterial stiffness contributes to cognitive decline. Finally, A+ CU and CU-Declining had similar Aβ times (e.g. ~7 years), yet CU displayed higher perfusion and longer pulse wave delays indicating superior tissue/vascular health. Current sample size is a limitation of this study.Acknowledgements
We gratefully acknowledge research support from GE Healthcare, and funding support from the Alzheimer's Association (AARFD-20-678095) and from NIH grants R01NS066982, P50-AG033514, and R01AG021155.
References
1. Bilgel M, An Y, Zhou Y, et al. Individual estimates of age at detectable amyloid onset for risk factor assessment. Alzheimer’s & Dementia. 2016;12(4):373-379. doi:10.1016/j.jalz.2015.08.166.
2. Koscik RL, Betthauser TJ, Jonaitis EM, et al. Amyloid duration is associated with preclinical cognitive decline and tau PET. Alzheimer’s & Dementia: Diagnosis, Assessment & Disease Monitoring. 2020;12(1):e12007. doi:10.1002/dad2.12007.
3. Jagust WJ, Landau SM. Temporal Dynamics of β-Amyloid Accumulation in Aging and Alzheimer Disease. Neurology. 2021;96(9):e1347. doi:10.1212/WNL.0000000000011524.
4. Villemagne VL, Burnham S, Bourgeat P, et al. Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: a prospective cohort study. The Lancet Neurology. 2013;12(4):357-367. doi:10.1016/S1474-4422(13)70044-9.
5. Jack CR, Wiste HJ, Weigand SD, et al. Predicting future rates of tau accumulation on PET. Brain. 2020;143(10):3136-3150. doi:10.1093/brain/awaa248.
6. Arvanitakis Z, Capuano AW, Leurgans SE, Bennett DA, Schneider JA. Relation of cerebral vessel disease to Alzheimer’s disease dementia and cognitive function in elderly people: a cross-sectional study. Lancet Neurol. 2016;15(9):934-943. doi:10.1016/S1474-4422(16)30029-1.
7. Zlokovic BV, Gottesman RF, Bernstein KE, et al. Vascular contributions to cognitive impairment and dementia (VCID): A report from the 2018 National Heart, Lung, and Blood Institute and National Institute of Neurological Disorders and Stroke Workshop. Alzheimers Dement. 2020 Dec;16(12):1714-1733. doi: 10.1002/alz.12157.
8. Sweeney MD, Sagare AP, Zlokovic BV. Blood–brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nature Reviews Neurology. 2018;14:133
9. Rivera-Rivera LA, Eisenmenger L, Cody KA, et al. Cerebrovascular stiffness and flow dynamics in the presence of amyloid and tau biomarkers. Alzheimer's & Dementia: Diagnosis, Assessment & Disease Monitoring. DOI: 10.1002/dad2.12253
10. Mestre H, Mori Y, Nedergaard M. The Brain’s Glymphatic System: Current Controversies. Trends in Neurosciences. 2020;43(7):458-466. doi:10.1016/j.tins.2020.04.003
11. Carare RO, Aldea R, Bulters D, et al. Vasomotion Drives Periarterial Drainage of Aβ from the Brain. Neuron 2020; 105: 400–401.
12. Koscik RL, Bruce P Hermann BP, Allison S, et al. Validity Evidence for the Research Category, "Cognitively Unimpaired - Declining," as a Risk Marker for Mild Cognitive Impairment and Alzheimer's Disease. Front Aging Neurosci. 2021 Jul 26;13:688478. doi: 10.3389/fnagi.2021.688478. eCollection 2021.
13. Johnson KM, Lum DP, Turski PA, et al. Improved 3D Phase Contrast MRI with Off-resonance Corrected Dual Echo VIPR. Magn Reson Med. 2008 Dec; 60(6): 1329–1336.
14. Ong F, Lustig M. SigPy: A Python Package for High Performance Iterative Reconstruction. 27th Annual ISMRM Meeting, Montreal, Canada, p. 4819.
15. Ying L, Sheng J. Joint image reconstruction and sensitivity estimation in SENSE (JSENSE). Magn Reson Med. 2007 Jun;57(6):1196-202.
16. Jimenez JE, Strigel RM, Johnson KM, et al. Feasibility of high spatiotemporal resolution for an abbreviated 3D radial breast MRI protocol. Magn Reson Med. 2018 Oct;80(4):1452-1466.
17. Loecher M, Schrauben E, Johnson KM, et al. Phase unwrapping in 4D MR flow with a 4D single-step laplacian algorithm. J Magn Reson Imaging 2016; 43: 833–842.
18. Schrauben E, Wåhlin A, Ambarki K, et al. Fast 4D flow MRI intracranial segmentation and quantification in tortuous arteries. J Magn Reson Imaging. 2015 Nov;42(5):1458-64.
19. Allison, S.L., Koscik, R.L., Cary, R.P., et al. Comparison of different MRI-based morphometric estimates for defining neurodegeneration across the Alzheimer's disease continuum. NeuroImage: Clin. 2019 23, 101895.
20. Patenaude, B., Smith, S.M., Kennedy, D., and Jenkinson M. A Bayesian Model of Shape and Appearance for Subcortical Brain NeuroImage, 56(3):907-922, 2011.
21. Schmidt P., Gaser C., Arsic M., et al. An automated tool for detection of FLAIR-hyperintense white-matter lesions in multiple sclerosis. Neuroimage. 2012;59:3774–3783.
22. Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. 2004 Mar;55(3):306-19. doi: 10.1002/ana.20009.
23. Betthauser TJ, Koscik RL, Jonaitis EM, et al. Amyloid and tau imaging biomarkers explain cognitive decline from late middle-age. Brain 2020; 143: 320–335.
24. Betthauser TJ, Koscik RL, Jonaitis EM, et al. Amyloid Time: Quantifying the onset of abnormal biomarkers and cognitive impairment along the Alzheimer’s disease continuum. 2021 Alzheimer's Association International Conference. July 27, 2021.
Figures
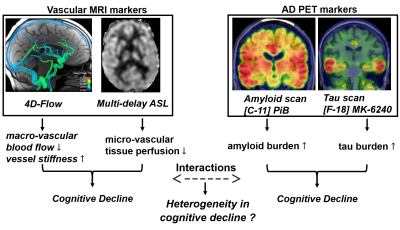
Figure 1: Schematic representation of neuroimaging techniques utilized to probe different vascular and AD biomarkers and how changes in these markers might impact cognition. Characterization of macro- and micro- vascular compartments was done using 4D-Flow and multi-delay ASL, while amyloid burden was quantified from PiB PET. Ongoing studies plan to include tau burden.

Table 1. Participant demographics and summary of Alzheimer’s disease biomarkers. A+/− represent β-amyloid positivity groups determined by 11C-PiB. In the amyloid negative (A-) groups there were no subjects with clinical dementia. Pairwise differences were assessed using ANOVA followed by post hoc analysis using the Tukey‐Kramer method (P<0.05 significance). Abbreviations: CU, cognitively unimpaired; APOE, apolipoprotein E; ICV, intracranial volume; PiB, 11C-Pittsburgh compound B; DVR, distribution volume ratio.

Table 2. Summary of vascular biomarkers. In the A+ groups there was no ASL data available for the 4.Dementia group. In the A- groups there were no subjects with clinical dementia. White matter hyperintensities were from T2-FLAIR, macro-vascular blood flow was from 4D-Flow and micro-vascular perfusion from transit time corrected ASL. Abbreviations: CU, cognitively unimpaired.
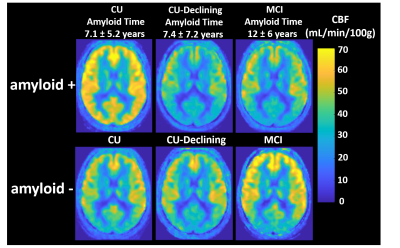
Figure 4. Averaged and normalized transit-time corrected CBF maps derived from ASL data for all participants. Amyloid positive (A+) cognitively unimpaired (CU) displayed significantly higher levels of perfusion in GM, WM and Hippocampus when compared to A+ CU-declining and mild cognitive impaired (MCI). Visually higher perfusion is strongly noticeable in the posterior aspect of the GM in A+ CU even after accumulating amyloid for the same amount of time than CU-Declining (~7 years). No significant perfusion differences were observed in A- groups.
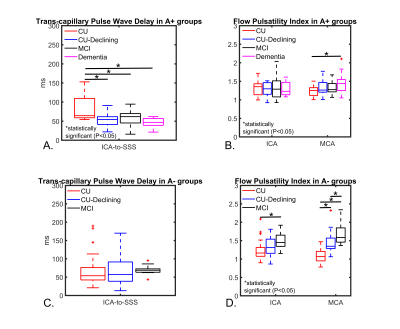
Figure 5. Trans-capillary pulse wave delay and flow pulsatility index (PI) derived from 4D-Flow data for all participants. In A+ groups, CU displayed significantly longer pulse wave delay when compared to other A+ groups. However, flow PI was similar in A+ CU subjects except for a significantly higher PI measured in the middle cerebral arteries (MCAs) of A+ dementia. A- groups displayed similar pulse wave delays; however, A- CU flow PI was significantly lower compared to CU-Declining and MCI in the internal carotid arteries (ICAs) and MCAs. Finally, A+ CU flow PI was higher than in A- CU.