0718
Towards an MRI-based Prediction of Neurofibrillary Tangles1Biomedical Engineering, Illinois Institute of Technology, Chicago, IL, United States, 2Rush Alzheimer's Disease Center, Rush University Medical Center, Chicago, IL, United States
Synopsis
The purpose of this work was to develop an MRI-based classifier of neurofibrillary tangles by combining ex-vivo MRI and detailed neuropathology in brain autopsies from a large number (N=878) of community-based older adults. The ex-vivo classifier was trained on volumetric, cortical thickness, subcortical shape, diffusion, and R2 measurements, as well as age and sex. The average AUC of the final classifier was 0.87. Future work will convert the ex-vivo classifier to in-vivo and test it in-vivo in independent cohorts of older adults.
Introduction
Neurofibrillary tangles, one of the hallmark pathologies of Alzheimer’s disease, are closely related to brain atrophy and cognitive decline 1. Even though tau PET tracers are now available, the search continues for biomarkers of tangles that are less invasive, cheaper, more accessible, and easier to use. Recent advances in blood-based biomarkers have identified promising markers 2 that even though may be less specific than PET, they combine several important advantages: they do not involve ionizing radiation, they are more accessible, easier to use, and cheaper. At the same time, most of the people that are candidates for tau PET biomarkers typically also undergo MRI, which is the safest imaging modality for assessing not only brain atrophy but also for collecting rich information on vascular disease, brain function, brain structural and functional connectivity, brain chemical characteristics, lesions, etc. Therefore, MRI-based prediction of neurofibrillary tangles would be highly desirable and is a field of active research 3. The purpose of this work was to develop an MRI-based classifier of neurofibrillary tangles by combining ex-vivo MRI and detailed neuropathology in brain autopsies from a large number of community-based older adults.Methods
Postmortem dataCerebral hemispheres from 878 older adults participating in three longitudinal, clinical-pathologic cohort studies of aging: the Rush Memory and Aging Project (MAP), the Religious Orders Study (ROS), and the Minority Aging Research Study (MARS) 4,5 (Fig. 1) were included in this work. All hemispheres were imaged at room temperature while immersed in 4% formaldehyde solution using clinical 3T MRI scanners, once within 24 hours postmortem and a second time approximately 30 days postmortem. Following the second ex-vivo MRI scan, all hemispheres underwent detailed neuropathologic examination. Neurofibrillary tangles were visualized with Bielschowsky silver stain, and counted and summarized to yield a Braak stage 6.
Ex-vivo classifier
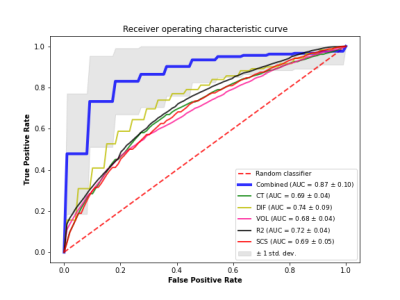
An SVM classifier with l2 regularization was trained to distinguish participants at a Braak stage of V-VI from those at a Braak stage of 0-IV, based on features extracted from ex-vivo MRI as well as demographic information (age, sex). The MRI features included volumetric, cortical thickness, subcortical shape, diffusion, and R2 measurements. When a feature contained multiple measurements per person i.e. cortical thickness measurements from multiple brain regions, the multiple measurements were used to train a separate model that generated a separate risk score, and that risk score was used as a feature in the final model. Multiple classifiers using the above features in isolation or in different combinations were tested to determine the classifier with the best performance. To assess performance in each case, stratified shuffle split cross-validation was repeated 100 times, each time using 80% of the data for training and 20% for testing, and the average area under the receiver operating characteristic curve (AUC) was computed. Because different groupings of features were available on different subgroups of the participants, the performance of the different classifiers was tested in a total of 74 participants (Fig. 2) that had measurements on all features. Yet the risk scores for the different features were constructed using all available data for that feature (e.g. the volumetric risk score was constructed based on data from all 878 participants).
Results
The average AUC of the classifier based on all MRI and demographic features was 0.87 with 82% mean sensitivity (SEN) and 77% mean specificity (SPE) (Fig. 3). In comparison, the average AUC of a classifier trained only on cortical thickness and demographics was 0.69 (SEN 67.1%, SPE 62.7%), only diffusion and demographics was 0.74 (SEN 74.3%, SPE 63.5%), only volumetrics and demographics was 0.68 (SEN 51.2%, SPE 75.8%), only R2 and demographics was 0.72 (SEN 66.3%, SPE 65.4%), only subcortical shapes and demographics was 0.69 (SEN 63.4%, SPE 64.6%) (Fig. 3).Discussion
The performance of the best performing ex-vivo classifier developed in this work (AUC=0.87) is rather encouraging. In comparison, recently published work combining in-vivo MRI and pathology data trained an MRI-based classifier of people at Braak stage V-VI vs. 0-II and achieved an AUC=0.69 3. Our next step will be to translate the ex-vivo classifier to in-vivo and test it in-vivo in independent cohorts of older adults. Successful completion of this work may result in a non-invasive classifier of neurofibrillary tangles which will allow refined participant selection in clinical trials for Alzheimer’s and will advance the development of targeted therapies.Acknowledgements
National Institute of Neurological Disorders and Stroke (NINDS) UH2-UH3NS100599
National Institute of Neurological Disorders and Stroke (NINDS) UF1NS100599
National Institute on Aging (NIA) R01AG064233
National Institute on Aging (NIA) R01AG067482
National Institute on Aging (NIA) R01AG017917
National Institute on Aging (NIA) R01AG015819
National Institute on Aging (NIA) RF1AG022018
National Institute on Aging (NIA) R01AG056405
National Institute on Aging (NIA) P30AG010161
National Institute on Aging (NIA) P30AG072975
References
1. Jack CR Jr, Knopman DS, Jagust WJ, et al. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12(2):207-216.
2. Chatterjee P, Pedrini S, Ashton NJ, et al. Diagnostic and prognostic plasma biomarkers for preclinical Alzheimer's disease. Alzheimers Dement. 2021 Sep 8.
3. Dallaire-Théroux C, Beheshti I, Potvin O, et al. Braak neurofibrillary tangle staging prediction from in vivo MRI metrics. Alzheimers Dement (Amst). 2019;11:599-609.
4. Barnes LL, Shah RC, Aggarwal NT, et al. The Minority Aging Research Study: ongoing efforts to obtain brain donation in African Americans without dementia. Curr Alzheimer Res. 2012 Jul;9(6):734-45.
5. Bennett DA, Buchman AS, Boyle PA, et al. Religious Orders Study and Rush Memory and Aging Project. J Alzheimers Dis. 2018;64(s1):S161-S189. doi:10.3233/JAD-179939.
6. Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239-59.
Figures
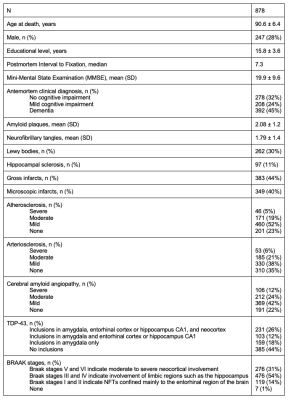
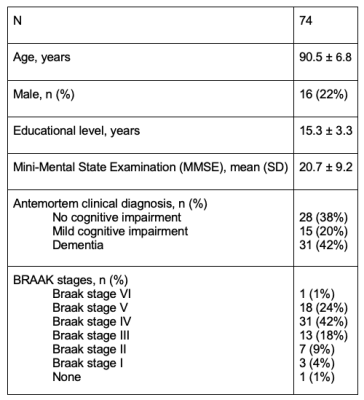
Figure 2: Demographics and characteristics of the participants used in the final model of ex-vivo classifier.