0716
Tracking developmental connectopathy in 22q11.2 deletion syndrome with cross-species fMRI1Italian Institute of Technology, Rovereto (TN), Italy, 2Italian Institute of Technology, Genova, Italy, 3Department of Biology, Unit of Cell and Developmental Biology, University of Pisa, Pisa, Italy, 4Department of Psychiatry and Biobehavioral Sciences and psychology Semel Institute for Neuroscience and Human Behavior, UCLA, Los Angeles, CA, United States
Synopsis
22q11.2 Deletion Syndrome (22q11DS), a major risk factor schizophrenia and autism, is often associated with disrupted brain connectivity. However, the developmental and neural underpinnings of brain connectopathy in 22q11DS remain unclear. Using mouse fMRI, we found that 22q11DS-related dysconnectivity exhibits a stereotypical developmental trajectory, with widespread functional hyperconnectivity in pre-pubertal mice reverting to focal fronto-hippocampal hypoconnectivity in adulthood. Notably, juvenile hyperconnectivity, but not adult hypoconnectivity, was completely rescued by a GSK3β antagonist. Finally, guided by our mouse model data, we identified a similar developmental trajectory in 22q11DS patients, hence corroborating the translational validity of these findings.
Introduction
22q11.2 deletion syndrome (22q11DS) is a genetic syndrome with high penetrance for developmental neuropsychiatric disorders such as autism spectrum disorders and schizophrenia1,2. Human imaging studies have shown alterations in functional connectivity across brain regions in individuals with 22q11DS3,4. These studies have promoted a conceptualization of 22q11DS as a ‘developmental dysconnectivity’ syndrome, possibly involving atypical circuit formation due to abnormal neurodevelopment. However, the neural underpinnings and developmental course of brain connectopathy in 22q11DS remain undetermined.To track the developmental trajectory in 22q11DS, here we longitudinally mapped resting-state fMRI (rsfMRI) connectivity and socio-cognitive behavior in LgDel mice, a well characterized mouse model of 22q11DS5,6. We found that 22q11DS is associated with prepubertal fMRI hyperconnectivity that reverts to focal hypoconnectivity in adult mice. We also found that a similar developmental trajectory is identifiable across development in 22q11DS patients. Finally, using pharmacological studies in the mouse, we linked juvenile hyperconnectivity (but not adult hypoconnectivity) to aberrant GSK3β signalling. Our data shed light on the course and determinants of brain dysconnectivity in 22q11DS.
Methods
Mouse studies: All experiments were carried out in accordance with Italian regulations governing animal welfare and protection (DL 26/2014). We used a cross-sectional treatment protocol with four cohorts of n=22 LgDel animals (mixed sexes). See Figure 1A for experimental timeline. Animal cohorts were: LgDel mice developmentally treated with the GSK3β inhibitor SB216763 (see 7); LgDel mice treated with vehicle; control (WT) littermates treated with SB216763; WT littermates treated with vehicle. SB216763 or vehicle were administered every second day from p7 to p27 as in7 (Figure 2A). rsfMRI-based functional connectivity was longitudinally mapped in juvenile (p33-p37) and adult (p105-p120) mice8. Functional images were acquired with a 7T MRI scanner using an EPI sequence with the following parameters: TR/TE 1000/15 ms, FOV 2.3 x 2.3 cm, flip angle 60°, matrix 98x98x18, slice thickness 550 µm, 1800 volumes. rsfMRI time series were analyzed by mapping long-range connectivity (as previously described9) followed by subsequent seed-based analyses12. Socio-cognitive functions were assessed with tasks for social preference and recognition, social reward and temporal order memory10,11. Human rsfMRI: Guided by mouse model studies, we performed long-range connectivity mapping and seed-based functional connectivity analyses in a sample of 96 22q11DS patients and healthy controls of an ongoing data collection effort at UCLA (see 12 for further details). Preprocessing was carried out as in8. The sample was divided into two groups based on participants’ age, resulting in a pre-pubertal cohort (n = 41, age range 6-12, 17 22q11DS patients) and an adult group (n = 55, age range 13-39, 32 22q11DS patients) .Results and discussion
rsfMRI mapping in mice revealed significant alterations in connectivity both at the juvenile and adult stages, with notable developmental differences in their direction and anatomical distribution. Specifically, we found evidence of generalized and robust hyperconnectivity in juvenile LgDel mice, an effect that reverted to focal fronto-hippocampal hypoconnectivity in adulthood (Figure 1). To determine the neural underpinnings of rsfMRI dysconnectivity, we probed whether inhibition of the GSK3β pathway7,13–15 could normalize aberrant fMRI coupling in LgDel mice, as prior research showed that this procedure may normalize fronto-hippocampal asynchrony in an analogous mouse model7.Notably, developmental Gsk3β kinase inhibition completely rescued juvenile hyperconnectivity, but did not normalize adult fronto-hippocampal rsfMRI hypoconnectivity (Figure 2B-D). The treatment also rescued cognitive dysfunction (Fig 3D), but no other socio-behavioral alterations assayed at both developmental timepoints. Interestingly, we found that connectivity changes were not predictive of impaired sociability (two-way ANOVA, genotype, p= 0.005), reward processing (t test, p = 0.04) or fronto-hippocampal cognitive functions (t test, p = 0.0004) assayed in the same mice across development (Figure 3A-D).
Finally, to probe whether a similar dysconnectivity signature would be identifiable in patients with 22q11DS, we investigated the developmental trajectory of rsfMRI connectivity in a human cohort. Guided by the mouse data and using identical analyses, we found evidence of hyperconnectivity in middle temporal gyrus (MTG) in 22q11DS children compared to controls (cluster corrected, cluster defining threshold t > 1.7, p < 0.05, one-tailed) and hypoconnectivity in hippocampus in 22q11DS adults compared to controls (Figure 4A). Further seed-based investigations revealed functional hypersynchronization between MTG and nodes from otherwise segregated networks (default mode network and central executive networks) in 22q11DS children compared to controls, indicative of broad hyperconnectivity. This effect was not present in the adult subsample (Figure 4B), in which we found instead decreased fronto-hippocampal coupling, together with hippocampal-cortical hypoconnectivity relative to controls (Figure 4C).
Conclusions.
Our data highlight developmental dysconnectivity in LgDel mice and an analogous, previously unrecognized developmental reconfiguration of connectivity in 22q11DS patients, recapitulating findings observed in the mouse model. We also report that these two signatures can be mechanistically segregated, with juvenile hyperconnectivity (but not adult hypoconnectivity), being linked to aberrant GSK3β signaling. The lack of a robust association between our imaging findings and corresponding behavioral deficits in the mouse suggests that the observed imaging phenotypes, while potentially useful biomarkers for disease progression, might be mechanistically distinct from the socio-cognitive dysfunction that characterizes 22q11DS.Acknowledgements
This work was supported by National Institute of Health (NIH, (1R21MH116473-01A1 to A. Gozzi, RO1 MH085953 to CEB).References
1. Drew LJ, Crabtree GW, Markx S, et al. The 22q11.2 microdeletion: Fifteen years of insights into the genetic and neural complexity of psychiatric disorders. Schizophrenia. 2011;29(3):259-281.
2. Gur RE, Bassett AS, McDonald-McGinn DM, et al. A neurogenetic model for the study of schizophrenia spectrum disorders: the International 22q11.2 Deletion Syndrome Brain Behavior Consortium. Mol Psychiatry. 2017;22(12):1664-1672.
3. Jalbrzikowski M, Villalon-Reina JE, Karlsgodt KH, et al. Altered white matter microstructure is associated with social cognition and psychotic symptoms in 22q11.2 microdeletion syndrome. Front Behav Neurosci. 2014;8:393.
4. Schreiner M, Forsyth JK, Karlsgodt KH, et al. Intrinsic Connectivity Network-Based Classification and Detection of Psychotic Symptoms in Youth With 22q11.2 Deletions. Cereb Cortex. 2017;27(6):3294-3306.
5. Merscher S, Funke B, Epstein JA, et al. TBX1 is responsible for cardiovascular defects in velo-cardio-facial/DiGeorge syndrome. Cell. 2001;104(4):619-629.
6. Meechan DW, Maynard TM, Tucker ES, et al. Modeling a model: Mouse genetics, 22q11. 2 Deletion Syndrome, and disorders of cortical circuit development. Prog Neurobiol. 2015;130:1-28.
7. Tamura M, Mukai J, Gordon JA, Gogos JA. Developmental inhibition of Gsk3 rescues behavioral and neurophysiological deficits in a mouse model of schizophrenia predisposition. Neuron. 2016;89(5):1100-1109.
8. Pagani M, Barsotti N, Bertero A, et al. mTOR-related synaptic pathology causes autism spectrum disorder-associated functional hyperconnectivity. Nat Commun. 2021;12(1):6084. doi:10.1038/s41467-021-26131-z
9. Cole MW, Pathak S, Schneider W. Identifying the brain’s most globally connected regions. Neuroimage. 2010;49(4):3132-3148.
10. Papaleo F, Yang F, Paterson C, et al. Behavioral, Neurophysiological, and Synaptic Impairment in a Transgenic Neuregulin1 (NRG1-IV) Murine Schizophrenia Model. J Neurosci Off J Soc Neurosci. 2016;36(17):4859-4875. doi:10.1523/JNEUROSCI.4632-15.2016
11. Huang H, Michetti C, Busnelli M, et al. Chronic and acute intranasal oxytocin produce divergent social effects in mice. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2014;39(5):1102-1114. doi:10.1038/npp.2013.310
12. Schleifer C, Lin A, Kushan L, et al. Dissociable Disruptions in Thalamic and Hippocampal Resting-State Functional Connectivity in Youth with 22q11.2 Deletions. J Neurosci. 2019;39(7):1301. doi:10.1523/JNEUROSCI.3470-17.2018
13. Mukai J, Dhilla A, Drew LJ, et al. Palmitoylation-dependent neurodevelopmental deficits in a mouse model of 22q11 microdeletion. Nat Neurosci. 2008;11(11):1302-1310.
14. Mukai J, Tamura M, Fénelon K, et al. Molecular substrates of altered axonal growth and brain connectivity in a mouse model of schizophrenia. Neuron. 2015;86(3):680-695.
15. Moutin E, Nikonenko I, Stefanelli T, et al. Palmitoylation of cdc42 promotes spine stabilization and rescues spine density deficit in a mouse model of 22q11. 2 deletion syndrome. Cereb Cortex. 2017;27(7):3618-3629.Figures
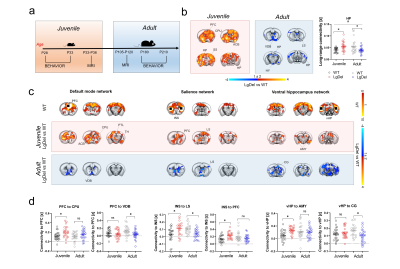
Figure 1. fMRI connectivity across development in LgDel mice a) Experimental timeline. b) Foci of long-range functional connectivity across development. c) Seed-based connectivity across development in LgDel mice with respect to control littermates. d) Regional quantification of connectivity differences between groups. [PFC, prefrontal cortex, INS, insular cortex, CPU, striatum, VDB, vertical diagonal band, vHP, ventral hippocampus, CG, cingulate cortex. # Age x Genotype p < 0.05].
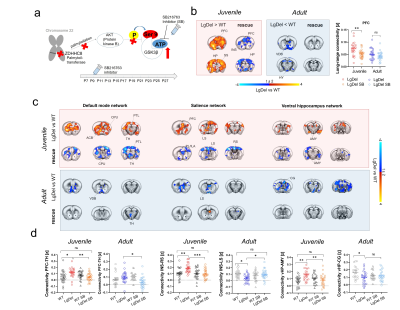
Figure 2. GSK3β-inhibition rescues juvenile hyperconnectivity in LgDel mice. a) Possible molecular cascade leading to GSK3β hyperactivity in LgDel mice. b) Treatment with SB216763 rescues long-range connectivity in juvenile but not adult mice. c) Seed based mapping and d) regional quantification of the rescue effect. [PFC, prefrontal cortex, INS, insular cortex, CPU, striatum, VDB, ventral diagonal band, vHP, ventral hippocampus, AMY, amygdala, CG, cingulate cortex, LS, lateral septum].
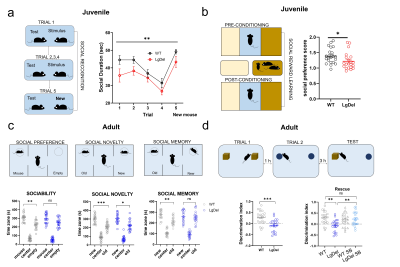
Figure 3. Socio-cognitive behavior across development. a) Habituation-dishabituation social interaction task in juvenile mice. b) Social conditioning place preference task in juvenile mice. c) Three chambered social approach task in adult mice. d) Temporal order memory task and pharmacological rescue in adult mice. Errors bars represent SEM. *p < 0.05; ** p< 0.01; *** p< 0.001.
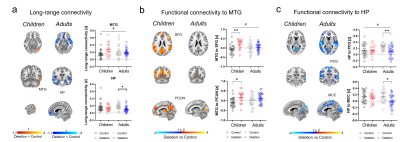
Figure 4. Connectivity across development in 22q11.2 patients. a) Long-range global connectivity in MTG and HP. b) Functional connectivity of MTG with DMN nodes (SFG and PCUN) across development. c) Functional connectivity of HP with PCG and MCC across development. [HP hippocampus, MCC midcingulate cortex, MTG middle temporal gyrus, PCUN precuneus, PCG precentral gyrus, SFG superior frontal gyrus. *p < 0.05, **p < 0.01. # Age x Genotype interaction p < 0.05. Error bars represent SEM.]