0711
Generalized approach of quadruple or quintuple-tuned RF coil setups for metabolic MRI throughout the body at 7 Tesla1UMC Utrecht, Utrecht, Netherlands, 2Tesla Dynamic Coils B.V., Zaltbommel, Netherlands, 3Technische Universiteit Eindhoven, Eindhoven, Netherlands
Synopsis
The 7T operating frequency of 1H and 19F RF transceivers is in far field regime, while for remaining nuclei these are in near-field regime. Consequently, the optimal setup for far field is based on antennas, while for near field these are based on loop coils. Owing to their intrinsic field orthogonality, these setups can be merged with low RF coupling and thus low penalty in efficiency as we demonstrated for a quintuple tuned head coil. Here we show that the design is generalized and can in principle be used for any body part, as demonstrated in brain, breast and extremity.
Introduction
Multi-nuclei magnetic resonance imaging (MRI) and spectroscopy (MRS) can provide unique information to conventional MRI. For example, Sodium (23Na) MRI has a huge potential in cancer diagnosis, prognosis, and immunotherapy-treatment-efficacy monitoring1,2. Phosphorus (31P) MRS is typically useful for energy metabolism study3. Carbon-13 (13C) MRS can measure neuroenergetics and neurotransmitter cycling by applying 13C-labeled precursors4. Multi-nuclei MRI and MRS raises more interest when it comes to higher fields like 7 Tesla where the typical lack of SNR is mitigated. In the presence of a dual-tuned body-coil for 23Na/13C and 31P, a generalized design can be used composed of a broadband antenna array as transceiver for 1H and 19F, merged with multiple dual-tuned 31P and 23Na receiver coil arrays, positioned close to the body part of interest, where the 23Na can be actively retuned to the 13C frequency. Next to a successful headcoil setup5 where these antennas and loops are bent over the curvature of a helmet, we show that these antennas and loops can be oriented along the main magnetic field (B0) to circumvent bilateral extremities or breasts when incorporating a mechanical breast frame. Bench measurements of X-nuclei channels (i.e. 31P, 23Na, 13C) as well as FDTD simulations at 1H frequency are performed to assess their SNR and B1+ efficiency when compared to single-tuned setups. Preliminary results from in vivo 1H MRI are obtained to show the advantage of using multi-transmit shimming while obtaining in vivo 13C, 23Na and 31P images.Methods
This quadruple-tuned array consists of an anterior part, a posterior part, and an interface platform, as shown in Figure 1. The anterior part is designed as four flexible compartments to closely fit the subject. The posterior part is designed as four surfaces to circumvent bilateral extremities or breasts. In each anterior compartment or on each posterior surface, a fractional dipole antenna is positioned5, which transmits and receives for 1H. Each dipole is placed in the middle of a surface-loop column, which is two loop coils overlapped along z-direction. The subject is placed feet first in supine position for extremity scan; head first in prone position for breast scan.The dipole antennas are tuned and matched at 298 MHz, which is the Larmor frequency of 1H at 7 Tesla. The surface loops’ circuit schematics refer to that of META head coil5: Each loop is normally dual tuned and matched for 31P and 23Na (i.e. 120.6 MHz and 78.8 MHz); The third resonance for 13C (i.e. 75 MHz) is achieved by active frequency shifting with a PIN diode; The decoupling between neighboring loops is achieved by overlapping; Dual-tuned wire-wound cable traps are applied at each RF port. The interface platform consists of sixteen RF splitters, thirty-two low-noise preamplifiers (i.e. sixteen for high-frequency , sixteen for low-frequency), thirty-two embedded digital receivers, a shift board, two DC splitters, and bazooka cable traps for all frequencies. Once the signals are received by the loops, they are distinguished by the RF splitters as high-frequency (120.8 MHz) or low-frequency (i.e. 74 MHz or 78.8 MHz), then guided into corresponding preamplifiers and receivers. The shift board provides the driver currents to set the diodes to the correct 23Na/13C mode. The DC splitters distribute DC power lines from the scanner to secure stable DC power supplies for the digital receivers. We use bazooka cable traps for cables carrying signals of all frequencies every quarter wavelength to prevent common-mode induced noise or signal reduction.
FDTD simulations (Sim4Life, ZMT, Switzerland) for 1H are performed with DUKE7 for extremity and a modified DUKE for breast. Since a regular female model is in supine position which is unsuitable for breast simulation, and a breast model lacks the trunk part, we combine DUKE with a breast model obtained from water-fat MR images (figure 3d). Bench measurements are done with a calibrated VNA (Copper Mountain Technologies, USA) and two weakly-coupled probes. System tests are done on a modified 7T whole-body MR system (Philips Healthcare, Best, NL).
Results
Head coil: Figure 2 shows the in vivo results of 1H, 31P, 23Na, and 13C MRI/MRSI from human brain, obtained with intrinsic losses of 15, 28, 29, 20% respectively when compared to non-coupled single tuned elements.Extremity or breast coil:
Proton: Figure 3 shows the simulated B1+ and SAR10g. With phase shimming between dipoles, 20 uT is achieved with 300 watts peak power per channel. Figure 4 shows in vivo scans of thighs of a healthy volunteer.
Phosphorus: Bench measurements show that the Q-factor ratio (i.e. Qloaded/Qunloaded) increases from 0.28 to 0.57 by going from a single-tuned 31P loop to a triple-tuned loop, meaning an intrinsic SNR loss8 of 23%.
Sodium and Carbon-13: Bench measurements indicate an SNR loss of 22% for 23Na, and 24% for 13C.
Discussion and Conclusion
We presented a quadruple-tuned breast/extremity coil using the same principle design of dipoles merged with multi-tuned receiver loops as used by the META headcoil. Promising preliminary results have been achieved where intrinsic losses caused by multi-tuning could be kept below 29%. These coil designs enable noninvasive biochemical studies using multiple nuclear species in the same scan session in principle anywhere on the body.Acknowledgements
I would like to thank Dutch Research Council (NWO) for funding the META-scan project.References
[1] Poku LO, Phil M, Cheng Y, Wang K, Sun X. 23Na-MRI as a noninvasive biomarker for cancer diagnosis and prognosis. J. Magn. Reson. Imaging. 2021;53(4):995-1014. doi:10.1002/jmri.27147
[2] Jafari SH, et al. Breast cancer diagnosis: Imaging techniques and biochemical markers. J Cell Physiol. 2018 Jul;233(7):5200-5213. doi: 10.1002/jcp.26379. Epub 2018 Jan 19. PMID: 29219189.
[3] Boss A, Heskamp L, Breukels V, Bains LJ, van Uden MJ and Heerschap A. Oxidative capacity varies along the length of healthy human tibialis anterior. J Physiol, 2018. 596: 1467-1483. https://doi.org/10.1113/JP275009
[4] Rothman DL, De Feyter HM, De Graaf RA, Mason GF, Behar KL. 13C MRS studies of neuroenergetics and neurotransmitter cycling in humans. NMR Biomed. 2011 Oct;24(8):943-57. doi: 10.1002/nbm.1772. Epub 2011 Aug 31. PMID: 21882281; PMCID: PMC3651027.
[5] Dai J, et al. A Quintuple-Tuned RF Coil for Whole Brain Multi-Nuclei Magnetic Resonance Imaging and Spectroscopy at 7T. ISMRM 2021, #0178. https://submissions2.mirasmart.com/ISMRM2021/ViewSubmission.aspx?sbmID=4452&validate=false
[6] Raaijmakers AJ, Italiaander M, Voogt IJ, Luijten PR, Hoogduin JM, Klomp DW and van den Berg CA. The fractionated dipole antenna: A new antenna for body imaging at 7 Tesla. Magn. Reson. Med. 2016; 75: 1366-1374. https://doi.org/10.1002/mrm.25596
[7] Christ A, Kainz W, Hahn EG, et al. The Virtual Family—development of surface‐based anatomical models of two adults and two children for dosimetric simulations. Phys Med Biol. 2010;55:N23–N38.
[8] Hayes CE. Noise performance of surface coils for magnetic resonance imaging at 1.5 T. Med. Phys., 1985. 12(5): 604-607.
Figures
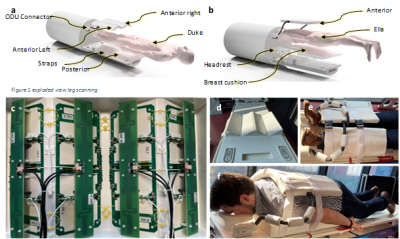
Figure 1 META extremity/breast coil (Tesla Dynamic Coils B.V., the Netherlands). a, b: 3D model of the coil housing. c: 1H dipoles and triple-tuned receiver loops. d: Posterior housing. e: Extremity loading condition. f: Breast loading condition.
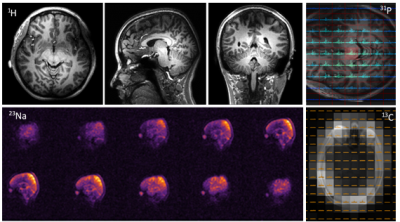
Figure 2 In vivo scan results by scanning a healthy volunteer with META head coil. Top left: three slices of 1H MRI with 3D TFE. Top right: 31P CSI FID. Bottom left: 23Na MRI by 3D FFE. Bottom right: 13C MRSI with natural abundance targeting lipids.
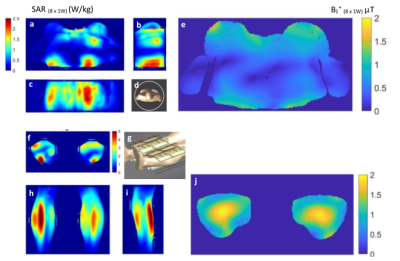
Figure 3 Simulated B1+ and SAR10 for 1H. a, b, c: Three views of simulated SAR10 for breast scans. d: Simulated model for breast scans. e: Simulated B1+ for breast scans. f, h, i: Three views of simulated SAR10 for extremity scans. g: Simulated model for extremity scans. j: Simulated B1+ for extremity scans.
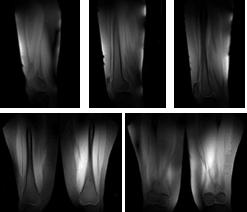
Figure 4 1H MRI of a healthy volunteer. Top: three slices from sagittal view. Bottom: two slices from coronal view.