0694
Novel non-invasive determination of parasagittal dural volume and cerebrospinal fluid flux: assessment of glymphatic clearance mechanisms1Neurology, Vanderbilt University Medical Center, Nashville, TN, United States, 2Radiology and Radiological Sciences, Vanderbilt University Medical Center, Nashville, TN, United States, 3Psychiatry and Behavioral Sciences, Vanderbilt University Medical Center, Nashville, TN, United States
Synopsis
Despite prior assumptions that the brain is devoid of lymphatic vessels, emerging evidence indicates that lymphatic vessels are present in the region alongside the superior sagittal sinus, the parasagittal dural (PSD) space, and could play a critical role in waste clearance or glymphatic physiology. However, limited methods are available for interrogating this space non-invasively in vivo. Here, in 62 healthy participants we apply novel deep learning algorithms in sequence with anatomical and phase contrast cerebrospinal fluid (CSF) flow assessments to test fundamental hypotheses regarding how PSD volume evolves across the lifespan and relates to measures of CSF flux and volume.
Introduction
The overall goal of this work is to apply novel deep learning algorithms in sequence with anatomical and phase contrast angiography through the cerebral aqueduct to test fundamental hypotheses regarding parasagittal dural (PSD) volume, which has been hypothesized to harbor cerebral lymphatic channels, and bulk cerebrospinal fluid (CSF) flow. The central nervous system has been assumed to be devoid of lymphatic vessels, however, following the proposal of the recent glymphatic system, preliminary evidence suggests that lymphatic vessels may occupy PSD spaces that surround the superior sagittal sinus1,2. MR studies utilizing exogenous contrast have suggested that transarachnoid molecular passage occurs in the PSD space, and this region may bridge the link between the glymphatic circulation within the brain and the dural lymphatic vessels. However, there are limited abilities to measure this space, and fundamental gaps persist in our knowledge of how variance in PSD volume relates to healthy aging and bulk CSF flow. We studied how CSF flow and volume relate to quantitative PSD volume measures. To enable this, we applied a novel deep learning algorithm to quantify the volume of the PSD non-invasively in vivo, in sequence with cardiac-phase corrected phase-contrast MRI measures of CSF flow through the cerebral aqueduct. The hypotheses to be investigated are PSD volume increases (Hypothesis-1) with healthy aging, and (Hypothesis-2) with bulk CSF flow through the cerebral aqueduct.Methods
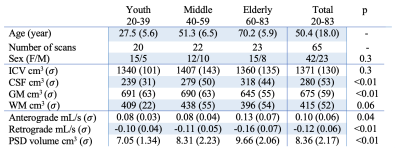
Participants (n=62) provided informed, written consent and were scanned at 3T (Philips Healthcare) using dual-channel body coil transmission and 32-channel phased array reception. Acqusition. 3D T1-weighted MPRAGE (spatial resolution=1x1x1mm), 3D T2-weighted VISTA (resolution=0.78x0.78x0.78mm), and retrospectively cardiac-corrected phase-contrast (PC) scan (venc=12 cm/s; measures per cardiac cycle=12) at the level of the aqueduct of Sylvius were acquired. Analysis. Intracranial (ICV) and brain tissue volumes were quantified using the brain mask computed by the advanced normalization tools3 from T1-weighted acquisitions. PSD volumes were computed using a segmentation method based on a combination of a fully connected neural network (trained using 20 manually segmented maps) and a voxel clustering based on a gaussian mixture model, both methods use only T2-weighted MRI as inputs. All segmentation maps were validated by a board-certified neuroradiologist (experience=12 years). CSF flux (ml/s) and velocity (cm/s) were quantified using the Q-Flow (Philips Healthcare)4. Hypothesis testing. We defined three models with age and sex as covariates to assess the relationship of PSD volume with (i) ICV, (ii) tissue volumes, and (iii) CSF flux. Hypotheses were tested using one-way ANOVA, corrected for multiple comparisons using false discovery rate (significance-criteria:q<0.05); correlations were estimated using Spearman’s rank coefficient, $$$\rho$$$.Results
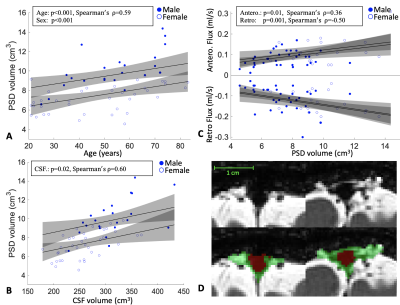
In total, 62 healthy participants were enrolled across the adult lifespan (age range = 20 to 83 years). Consistent with Hypothesis-1, PSD volume was observed to increase directly with age: p<0.001 (q-value=0.009) and $$$\rho$$$=0.59. A significant sex relationship was observed, whereby PSD volume was higher in males vs. females (p<0.001). ICV and PSD volume were not significantly related to PSD volume when sex was included as a covariate. Regarding Hypothesis-2 and to understand whether tissue or CSF volumes related to PSD volume, we found no evidence of a correlation between PSD volume and gray matter or white matter volume. PSD volume was positively correlated with CSF volume: p=0.02 (q-value=0.04) and $$$\rho$$$=0.6. Furthermore, a significant correlation between PSD volume and CSF flux for both directionalities was observed. Our model indicates that anterograde CSF flux correlates positively with PSD volume with p=0.01 (q-value=0.04), and $$$\rho$$$=0.36. Retrograde CSF flux showed stronger negative correlation, with a p=0.001 (q-value=0.004) and $$$\rho$$$=-0.50.Discussions
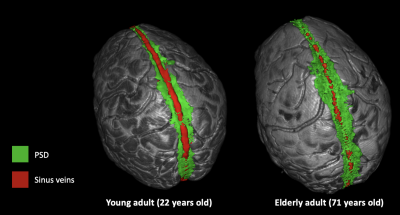
To our knowledge, this is the first time that the PSD volume, the space hypothesized to harbor cerebral lymphatic vessels, has been related to bulk CSF flow. Determination of this relationship was enabled by sequential incorporation of novel deep learning algorithms applied to high spatial resolution anatomical MRI data in sequence with re-parameterized quantitative phase-contrast CSF flow angiography. The technique we use allows for clear distinction of the PSD from both the adjacent subarachnoid space as well as the superior sagittal sinus and feeding cortical veins, and importantly, this method obviates the need for contrast-enhanced imaging which should make the approach feasible in a range of research studies. We observed PSD hypertrophy in relation to normal aging, CSF volume, and CSF flux in the cerebral aqueduct. Our study also supports that PSD volumes are not significantly related to brain parenchymal volume or ICV. This implies that increased PSD cannot be explained simply by tissue volume loss.Conclusions
We applied novel deep learning algorithms in sequence with phase-contrast angiography of CSF flow to test hypotheses regarding PSD volume hypertrophy and bulk CSF flow. Findings are consistent with PSD volume correlating with CSF volume and bulk CSF flux and increasing across the adult lifespan. Importantly, the methods used can be applied non-invasively in vivo and should provide new tools for interrogating CSF dynamics and potentially glymphatic dysfunction. Findings provide further evidence of an organized system of CSF circulation and metabolism and suggest a complex physiologic interplay between bulk CSF flow and possible cerebral lymphatic channels.Acknowledgements
This study was supported by NIH/NIA 5R01AG062574 and NIH/NCCIH 1R01AT011456.References
1. Absinta, M. et al. Human and nonhuman primate meninges harbor lymphatic vessels that can be visualized noninvasively by MRI. Elife 6, (2017).
2. Ringstad, G. & Eide, P. K. Cerebrospinal fluid tracer efflux to parasagittal dura in humans. Nat. Commun. 11, (2020).
3. Avants, B. B. et al. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage 54, 2033–2044 (2011).
4. A. Najafi, T. D. J. Sartoretti, C. A. Binkert, S. Sartoretti-Schefer & M. Wyss. CSF flow quantification in the cerebral aqueduct using phase contrast MR - How to do it properly. in European society of radiology (2018).
Figures