0676
Deuterium magnetic resonance spectroscopy using 2H-pyruvate allows in vivo imaging of tumor burden and response to therapy1Radiology and Biomedical Imaging, UCSF, San Francisco, CA, United States, 2Neurological Surgery, UCSF, San Francisco, CA, United States
Synopsis
Telomerase reverse transcriptase (TERT) is essential for tumor proliferation and is an attractive therapeutic target. Non-invasive methods of imaging TERT can report on tumor proliferation and response to therapy. Here, we show that 2H-MRS following administration of [U-2H]pyruvate non-invasively monitors TERT expression in clinically relevant patient-derived glioma models. Importantly, imaging TERT using [U-2H]pyruvate provides a readout of response to targeted TERT inhibitors and standard of care chemotherapy in vivo, at early timepoints that precedes MRI-detectable anatomical alterations. Clinical translation of our results can enable non-invasive assessment of TERT expression in vivo and, thereby, aid in imaging response to anti-cancer therapies.
Introduction
Most tumors, including high-grade glioblastomas (GBMs) and low-grade oligodendrogliomas (LGOGs) achieve immortality by reactivation of the expression of telomerase reverse transcriptase (TERT), which is silenced in normal somatic cells1,2. Since TERT expression is essential for tumor immortality, TERT can serve as a biomarker of tumor proliferation and provide a readout of response to therapy1,2. However, non-invasive methods of imaging TERT are lacking.2H-magnetic resonance spectroscopy (2H-MRS) is a novel method of monitoring metabolic fluxes in vivo3,4. Previous studies have linked TERT expression to elevated NADH, which is essential for the conversion of pyruvate to lactate5. Therefore, the goal of this study was to determine whether [U-2H]pyruvate can be used to non-invasively monitor TERT expression in vivo.
Methods
Cell culture: Patient-derived GBM (GBM1, GBM6) and LGOG (BT88) cells were maintained as described previously6-9. siRNAs against human TERT and non-targeting siRNA pool were used to transiently silence TERT expression5.2H-MRS of live cells: Cells were incubated in media containing 10mM [U-2H]pyruvate for 72h. Live cells were suspended in saline and 2H-MR spectra acquired using a 16mm surface coil on a Varian 14.1T scanner using a pulse-acquire sequence (TR=260ms, NA=2500, complex points=512, flip angle=64o, spectral width=2kHz). Data analysis was performed using Mnova. Lactate peak integrals were converted to mM concentration using the natural abundance HDO signal (12.8mM)3 collected from a vial containing saline.
MRI: Orthotopic tumors were generated by intracranial implantation of 3x105 cells10-12. Once tumors reached a volume of 27.0±7.8mm3, mice were treated with vehicle-control (saline), temozolomide (TMZ; 50mg/kg) or 6-thio-2'-deoxyguanosine (6-thio-dG; 50mg/kg) daily via intraperitoneal injection. T2-weighted MRI was performed on a Varian 14.1T scanner equipped with a single-channel 1H volume coil using a spin-echo sequence (TE/TR=20/1200ms, FOV=30x30 mm2, matrix=256x256, slice thickness=1mm, NA=4)10-12.
2H-MRS/I in vivo: 2H-MRS/I studies were performed using a 16mm 2H surface coil. A bolus of 450mg/kg [U-2H]pyruvate was injected via a tail-vein catheter and 2H-MR spectra acquired using a non-selective sequence (TR=500ms, spectral width=2kHz, number of points=512, flip angle=64º, temporal resolution=4min 10s). For spatial localization, a 2D chemical shift imaging (CSI) sequence with TE/TR=1.35/250ms, FOV=30x30x8mm3, 128 points, 2.5kHz spectral width and NA=20 resulting in a nominal voxel size of 112.5μl was used. Non-localized 2H-MR spectra were analyzed using Mnova. Lactate concentrations were determined by correcting peak integrals for saturation and normalizing to pre-injection HDO3. CSI data was analyzed using in-house Matlab codes and lactate signal-to-noise ratio (SNR) maps were derived. The SNR of 2H-lactate was assessed in a 10.99mm3 region of interest (ROI) from tumor and contralateral normal brain.
Statistical analysis: Results are expressed as mean ± standard deviation. Statistical significance was assessed using an unpaired two-tailed Student’s t-test with p<0.05 considered significant.
Results and Discussion
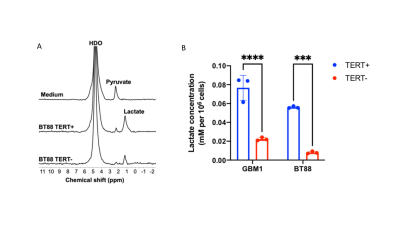
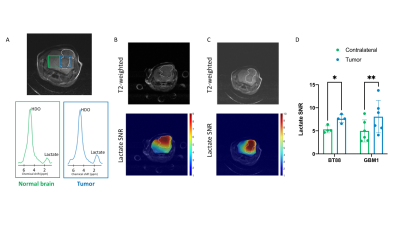
[U-2H]pyruvate monitors TERT expression First, we monitored lactate production from [U-2H]pyruvate in live cells. 2H-MR spectra from neat medium, prior to incubation with cells, showed peaks for HDO and [U-2H]pyruvate (Fig. 1A). 2H-MR spectra acquired from live cells incubated with media containing [U-2H]pyruvate showed lactate production with significantly higher levels in TERT+ cells relative to TERT- (Fig. 1A-1B), linking lactate production from [U-2H]pyruvate to TERT expression in glioma cells.[U-2H]pyruvate allows assessment of tumor burden in vivo Next, we performed 2D CSI in mice bearing orthotopic BT88 or GBM1 tumors. Representative spectra from tumor and contralateral normal brain voxels in a mouse bearing an orthotopic GBM1 tumor xenograft are shown in Fig. 2A. Importantly, lactate production from [U-2H]pyruvate delineated tumor from contralateral normal brain in both BT88 and GBM1 models (Fig. 2B-2D).
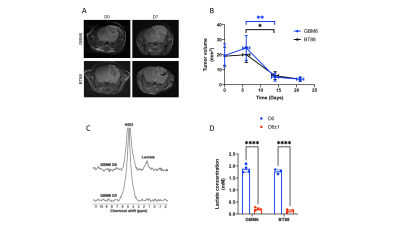
Lactate production from [U-2H]pyruvate is an early biomarker of response to therapy in vivo We then examined the ability of [U-2H]pyruvate to report on response to TERT inhibition in vivo. 6-thio-2'-deoxyguanosine (6-thio-dG) is an analog of the TERT substrate 6-thio-dGTP that induces telomere uncapping in TERT+ cancer cells and is in clinical trials for solid tumors13,14. Mice bearing GBM6 or BT88 tumors were treated with 6-thio-dG and [U-2H]pyruvate metabolism examined at day 0 and day 6±1. 6-thio-dG induced tumor shrinkage, an effect that was significant starting day 14±1 for both models (Fig. 3A-3B). Importantly, lactate production from [U-2H]pyruvate was significantly reduced at day 7, when no alteration in tumor volume could be detected by MRI (Fig. 3C-3D).
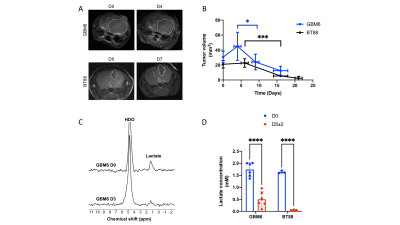
TERT is a biomarker of tumor proliferation and imaging TERT has the potential to report on response to cytocidal agents1,2. We, therefore, examined whether [U-2H]pyruvate reports on response to TMZ, which is standard of care for glioma patients15,16, in mice bearing orthotopic GBM6 or BT88 xenografts. As shown in Fig. 4A-4B, TMZ induced tumor shrinkage by day 9±1 in the GBM6 model and day 16±2 in the BT88 model. Importantly, [U-2H]pyruvate metabolism to lactate was significantly reduced at day 5±2 prior to tumor shrinkage (Fig. 4C-4D).
Conclusions
Our study, for the first time, indicates that [U-2H]pyruvate has the potential to assess TERT expression and to provide a readout of response to therapy, prior to MRI-detectable volumetric alterations, in clinically relevant patient-derived glioma models in vivo. Importantly, since 2H-MRS can be implemented on clinical scanners, our findings can be rapidly translated to the clinic and provide a novel, method of integrating critical genomic information, i.e., TERT, into glioma patient management.Acknowledgements
This study was supported by NIH R01CA239288, Department of Defense W81XWH201055315 and UCSF Brain Tumor Center Loglio and NICO initiatives.References
- Bell, R. J. et al. Understanding TERT Promoter Mutations: A Common Path to Immortality. Molecular cancer research : MCR 14, 315-323, doi:10.1158/1541-7786.mcr-16-0003 (2016).
- Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646-674, doi:10.1016/j.cell.2011.02.013 (2011).
- De Feyter, H. M. et al. Deuterium metabolic imaging (DMI) for MRI-based 3D mapping of metabolism in vivo. Sci Adv 4, eaat7314 (2018).
- Lu, M., Zhu, X. H., Zhang, Y., Mateescu, G. & Chen, W. Quantitative assessment of brain glucose metabolic rates using in vivo deuterium magnetic resonance spectroscopy. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 37, 3518-3530 (2017).
- Viswanath, P. et al. Non-invasive assessment of telomere maintenance mechanisms in brain tumors. Nature communications 12, 92, doi:10.1038/s41467-020-20312-y (2021).
- Bell, R. J. et al. Cancer. The transcription factor GABP selectively binds and activates the mutant TERT promoter in cancer. Science 348, 1036-1039, doi:10.1126/science.aab0015 (2015).
- Mancini, A. et al. Disruption of the beta1L Isoform of GABP Reverses Glioblastoma Replicative Immortality in a TERT Promoter Mutation-Dependent Manner. Cancer cell 34, 513-528 e518 (2018).
- Sarkaria, J. N. et al. Use of an orthotopic xenograft model for assessing the effect of epidermal growth factor receptor amplification on glioblastoma radiation response. Clinical cancer research : an official journal of the American Association for Cancer Research 12, 2264-2271, doi:10.1158/1078-0432.Ccr-05-2510 (2006).
- Kelly, J. J. et al. Oligodendroglioma cell lines containing t(1;19)(q10;p10). Neuro-oncology 12, 745-755, doi:10.1093/neuonc/noq031 (2010).
- Batsios, G. et al. PI3K/mTOR inhibition of IDH1 mutant glioma leads to reduced 2HG production that is associated with increased survival. Sci Rep 9, 10521, doi:10.1038/s41598-019-47021-x (2019).
- Viswanath, P. et al. Mutant IDH1 gliomas downregulate phosphocholine and phosphoethanolamine synthesis in a 2-hydroxyglutarate-dependent manner. Cancer & metabolism 6, 3, doi:10.1186/s40170-018-0178-3 (2018).
- Viswanath, P. et al. 2-hydroxyglutarate-mediated autophagy of the endoplasmic reticulum leads to an unusual downregulation of phospholipid biosynthesis in mutant IDH1 gliomas. Cancer research, doi:10.1158/0008-5472.can-17-2926 (2018).
- Mender, I., Gryaznov, S., Dikmen, Z. G., Wright, W. E. & Shay, J. W. Induction of telomere dysfunction mediated by the telomerase substrate precursor 6-thio-2'-deoxyguanosine. Cancer discovery 5, 82-95, doi:10.1158/2159-8290.Cd-14-0609 (2015).
- Sengupta, S. et al. Induced Telomere Damage to Treat Telomerase Expressing Therapy-Resistant Pediatric Brain Tumors. Molecular cancer therapeutics 17, 1504-1514, doi:10.1158/1535-7163.Mct-17-0792 (2018).
- Weller, M. et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol, doi:10.1038/s41571-020-00447-z (2020).
- Stupp, R. et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. The New England journal of medicine 352, 987-996, doi:10.1056/NEJMoa043330 (2005).
Figures