0674
Oral delivery of 2H-labeled fumarate for deuterium magnetic resonance spectroscopic imaging of tumor cell death in vivo1CRUK CI, University of Cambridge, Cambridge, United Kingdom, 2Department of Radiology, University of Cambridge, Cambridge, United Kingdom, 3Department of Chemistry, University of Cambridge, Cambridge, United Kingdom, 4Department of Biochemistry, University of Cambridge, Cambridge, United Kingdom
Synopsis
Cell death is an important imaging target for assessing early tumour treatment response and the effectiveness of therapy. We show here that 2H-labelled fumarate can be administered orally to detect cell death and assess early tumour treatment response in a subcutaneous lymphoma (EL4) model. Following oral gavage, 2H spectra were acquired from tumors with a time resolution of 5 min. Within 48h after chemotherapeutic drug (etoposide) treatment the tumor malate/fumarate signal ratios increased similarly to those measured after intravenous injection.
Introduction
Cell death is an important imaging target for assessing early tumour treatment response and the effectiveness of therapy. The degree of tumour cell death can be a predictive indicator of patient outcome. We have shown that deuterium MRI can be used to detect cell death and assess early tumour treatment response by measuring an increase in the rate of malate production following an intravenous 2H-fumarate injection1. Fumarate is hydrated in a reaction catalysed by the enzyme fumarase to produce malate. Loss of plasma membrane integrity during cell necrosis results in fumarate rapidly gaining access to the enzyme and an increased rate of malate production1,2. Here, we investigated delivery of labelled fumarate by oral gavage.Materials and Methods
EL4 lymphoma-bearing mice were fasted for 1 h before oral administration (200 µl) of 2H-labeled fumarate (2g/kg bodyweight) through a blunt gavage needle without anaesthesia. Following oral administration, the animals were anaesthetised and imaged as described previously1,3. 2H MR spectroscopic images were acquired using a surface coil with a fast 3D deuterium MRI pulse sequence with a time resolution of 5 minutes, and a spatial resolution of 3 x 3 x 9 mm, before and 48 h after treatment with a chemotherapeutic drug (etoposide). 2H spectra were also acquired.Results
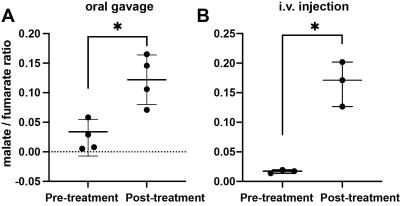
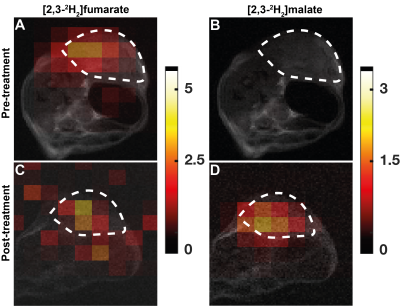
The conversion of [2,3-2H2]fumarate to [2,3-2H2]malate in implanted EL4 tumors in vivo was assessed at 30 min following oral gavage of labelled fumarate by EL4 tumor-bearing mice. A total of 13 spectra were acquired over 65 minutes. Both 2H-labelled fumarate and malate were detectable in the tumor tissue (Figure 2) and the malate/fumarate signal ratios post drug treatment increased similarly to those measured after intravenous injection, from 0.022 ± 0.03 to 0.12 ±0.04 (P=0.023, n=4) and 0.016 ± 0.02 to 0.16 ± 0.14 (P=0.0024, n=3), respectively (Figure 1).Discussion
Deuterium metabolic imaging (DMI) with [2,3-2H2]fumarate has potential for the quantitative assessment of tumor cell death in vivo. Delivering the 2H-labelled fumarate orally may improve clinical acceptability of the technique. The tumor malate/fumarate signal ratio observed after oral delivery of the labelled fumarate showed a similar increase post-drug treatment to that observed following i.v. injection.Conclusion
2H MR imaging of 2H-fumarate metabolism, where the fumarate is administered orally, can be used to non-invasively assess tumour cell death post drug treatment in a subcutaneous murine lymphoma model.Acknowledgements
The work was supported by grants from Cancer Research UK (C197/ A17242, C197/A16465, C9685/A25177). FH is in receipt of a Cambridge European Scholarship from the Cambridge Trust.References
1. Hesse F, Somai V, Kreis F, et al. Monitoring tumor cell death in murine tumor models using deuterium magnetic resonance spectroscopy and spectroscopic imaging. Proc Natl Acad Sci U S A 2021;118(12).
2. Gallagher FA, Kettunen MI, Hu D-E, et al. Production of hyperpolarized [1,4-13C2]malate from [1,4-13C2]fumarate is a marker of cell necrosis and treatment response in tumors. Proceedings of the National Academy of Sciences 2009;106(47):19801-19806.
3. Kreis F, Wright AJ, Hesse F, et al. Measuring Tumor Glycolytic Flux in Vivo by Using Fast Deuterium MRI. Radiology 2020;294(2):289-296.
Figures