0664
Improving Efficiency of Ultra-short Echo Time MRI in Neonatal Lungs with a FLORET Spiral Trajectory1Department of Radiology, University of Iowa, Iowa City, IA, United States, 2Department of Medical Physics, University of Wisconsin, Madison, Madison, WI, United States, 3Division of Pulmonary Medicine, Cincinnati Children's Hospital, Cincinnati, OH, United States, 4Department of Radiology, Cincinnati Children's Hospital, Cincinnati, OH, United States, 5Department of Pediatrics, University of Cincinnati, Cincinnati, OH, United States, 6Center for Pulmonary Imaging Research, Cincinnati Children's Hospital, Cincinnati, OH, United States, 7POLARIS, Imaging Sciences, Department of Infection, Immunity and Cardiovascular Disease, University of Sheffield, Sheffield, United Kingdom, 8Department of Radiology, University of Cincinnati, Cincinnati, OH, United States, 9Department of Physics, University of Cincinnati, Cincinnati, OH, United States
Synopsis
State-of-the-art structural pulmonary MRI of neonatal intensive care unit patients has previously been performed using 3D radial UTE. Performance is high yet scan times can be 15 minutes or more due to the low sampling efficiency of radial trajectories. Here we explore FLORET UTE in this application and demonstrate comparable image quality to the state-of-the-art in approximately 25% of the scan time. Lung parenchymal SNR is maintained, and the spiral trajectory provides a higher parenchymal signal relative to muscle. Shorter scan times reduce the opportunity for bulk motion and risk to this sensitive population.
Introduction
Radial 3D ultrashort echo time (UTE) MRI has been developed over the past several years as a tool for clinical evaluation of lung disease in infants within the neonatal intensive care unit (NICU)1–7. Importantly, the scan is robust to motion and provides self-navigation for retrospective respiratory gating8, allowing it to be performed during quiet breathing. Despite its promise, the scan times are long due to the requirement for very high isotropic 3D resolution (<1mm) in these patients’ small anatomies and the low sampling efficiency of 3D radial trajectories. Advances in 3D spiral UTE-capable sequences, specifically FLORET9–11, present an opportunity to maintain both image quality and retrospective gating capabilities while significantly reducing scan times. In this work, we compare FLORET to 3D radial UTE in a cohort of NICU patients suffering from lung disease of prematurity (bronchopulmonary dysplasia, BPD).Methods
All studies were approved by our institutional review board, HIPAA compliant, and conducted with parental consent. Seven neonatal patients underwent MRI in the NICU (5 female, 2 male, postmenstrual age at birth 26.2 ± 3.0 wks, postmentrual age at MRI 40.2 ± 2.4 wks) on a 1.5T scanner designed for neonatal imaging12,13, using a body coil. All patients were imaged using both 3D radial UTE and FLORET during a single exam. General acquisition parameters were: 180mm x 180mm x 180mm FOV, 0.703mm isotropic resolution, 5° flip angle, and 250kHz readout bandwidth. Specific 3D radial acquisition parameters were: TE/TR = 0.16/5.0ms, 200,000 radial views, 2ms readout duration, and 16:44 min scan time. Specific FLORET acquisition parameters were: TE/TR = 0.22/7.0ms, 40,000 spiral views, 2.5ms readout duration, 1 hub, 90° hub alpha, and 4:41 min scan time. Retrospective respiratory gating with 50% data acceptance was performed for both trajectories using the signal at the center of k-space as a motion navigator8. Regions of interest (ROIs) were selected in normal appearing lung and adjacent muscle tissue to measure mean lung and muscle signal, respectively. Standard deviation of the noise was estimated from an ROI in the background. Signal-to-noise ratio (SNR) in the lung and muscle were compared between both acquisition schemes and respiratory gating regimes (gated vs. ungated). The ratio of lung parenchyma to muscle signal was similarly compared. All comparisons were tested for statistical significance using paired t-tests.Results
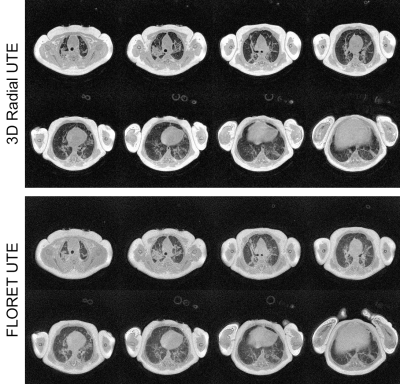
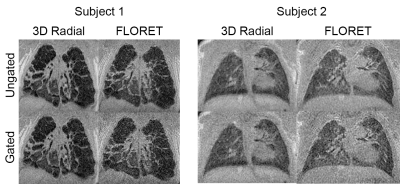
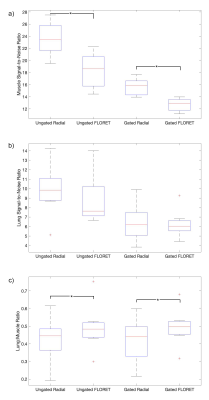
A series of axial slices are shown in a representative case in Figure 1 without respiratory gating. The visualization of pulmonary pathology is comparable in both acquisitions, with lower overall SNR yet higher relative parenchymal signal in the FLORET images. In Figure 2, coronal slices from 3D radial and FLORET are shown for 2 different patients with and without respiratory gating. These demonstrate the blurring effect of respiratory motion using both acquisitions and the capability to reduce motion blurring retrospectively. Quantitative comparisons of SNR show that the 3D radial has significantly greater SNR in muscle tissue both with gating (P=1e-5) and without (P=2e-5). However, no significant differences in lung parenchymal SNR between 3D radial and FLORET were found (without gating: P=0.34, with gating: P=0.46). This dichotomy is reflected in the lung-to-muscle signal ratio, which is significantly greater in FLORET compared to 3D radial (without gating: P=0.04, with gating: P=0.008). Boxplots of these metrics are provided in Figure 3, and the means and standard deviations of these values are summarized in Table 1. It is worth noting that applying retrospective respiratory gating similarly reduced SNR in both 3D radial and FLORET, with a reduction in SNR of approximately 67% at 50% data acceptance. Finally, retrospective gating did not change the lung-to-muscle tissue ratio, as expected.Discussion
It is expected that the SNR in muscle (long T2*) is lower using FLORET, given the factor of ~4 reduction in total data acquired, and thus scan time. Notably, a fully sampled FLORET (4:41 min scan time) has higher lung SNR and muscle SNR than a 50% sampled 3D radial (8:22 min effective scan time), so significant efficiency appears to be gained using the FLORET trajectory. Furthermore, the shorter acquisition does not appear to qualitatively hinder visualization of parenchymal structure. Lung parenchymal SNR is relatively equivalent between the radial and spiral acquisition schemes likely due to higher parenchymal signal using the FLORET trajectory. The increased parenchymal signal observed is possibly related to the lower radial sampling bandwidth (i.e. radial k-space velocity) of the spiral readout near the center of k-space. As a final note, there is potentially noise in the comparisons associated with inconsistent compliance of the infants across scans, even over the course of a single exam.Conclusion
We have demonstrated the feasibility of imaging NICU patients with BPD using a FLORET UTE sequence that performs comparably to the state-of-the-art 3D radial acquisition, while requiring approximately 25% of the scan time.Acknowledgements
The authors would like to acknowledge financial support from The Hartwell Foundation, GE Healthcare, The Perinatal Institute at Cincinnati Children’s Hospital Medical Center, and NIH P01 HL070831.References
1. Hahn AD, Higano NS, Walkup LL, et al. Pulmonary MRI of neonates in the intensive care unit using 3D ultrashort echo time and a small footprint MRI system. J Magn Reson Imaging. 2017;45(2). doi:10.1002/jmri.253942.
2. Higano NS, Spielberg DR, Fleck RJ, et al. Neonatal pulmonary magnetic resonance imaging of bronchopulmonary dysplasia predicts short-term clinical outcomes. Am J Respir Crit Care Med. 2018;198(10). doi:10.1164/rccm.201711-2287OC3.
3. Bates AJ, Higano NS, Hysinger EB, et al. Quantitative Assessment of Regional Dynamic Airway Collapse in Neonates via Retrospectively Respiratory-Gated 1 H Ultrashort Echo Time MRI. J Magn Reson Imaging. 2019;49(3). doi:10.1002/jmri.262964.
4. Higano NS, Fleck RJ, Spielberg DR, et al. Quantification of neonatal lung parenchymal density via ultrashort echo time MRI with comparison to CT. J Magn Reson Imaging. 2017;46(4). doi:10.1002/jmri.256435.
5. Higano NS, Bates AJ, Tkach JA, et al. Pre- and post-operative visualization of neonatal esophageal atresia/tracheoesophageal fistula via magnetic resonance imaging. J Pediatr Surg case reports. 2018;29:5-8. doi:10.1016/j.epsc.2017.10.0016.
6. Yoder LM, Higano NS, Schapiro AH, et al. Elevated lung volumes in neonates with bronchopulmonary dysplasia measured via MRI. Pediatr Pulmonol. 2019;54(8):1311-1318. doi:10.1002/ppul.243787.
7. Hysinger EB, Bates AJ, Higano NS, et al. Ultrashort Echo-Time MRI for the Assessment of Tracheomalacia in Neonates. Chest. 2020;157(3):595-602. doi:10.1016/j.chest.2019.11.0348.
8. Higano NS, Hahn AD, Tkach JA, et al. Retrospective respiratory self-gating and removal of bulk motion in pulmonary UTE MRI of neonates and adults. Magn Reson Med. 2017;77(3). doi:10.1002/mrm.262129.
9. Pipe JG, Zwart NR, Aboussouan EA, Robison RK, Devaraj A, Johnson KO. A new design and rationale for 3D orthogonally oversampled k-space trajectories. Magn Reson Med. 2011;66(5):1303-1311. doi:10.1002/mrm.2291810.
10. Robison RK, Anderson AG, Pipe JG. Three-dimensional ultrashort echo-time imaging using a FLORET trajectory. Magn Reson Med. 2017;78(3):1038-1049. doi:10.1002/mrm.2650011.
11. Willmering MM, Robison RK, Wang H, Pipe JG, Woods JC. Implementation of the FLORET UTE sequence for lung imaging. Magn Reson Med. 2019;82(3):1091-1100. doi:10.1002/mrm.2780012.
12. Tkach JA, Merhar SL, Kline-Fath BM, et al. MRI in the neonatal ICU: initial experience using a small-footprint 1.5-T system. AJR Am J Roentgenol. 2014;202(1):W95-W105. doi:10.2214/AJR.13.1061313.
13.Tkach JA, Hillman NH, Jobe AH, et al. An MRI system for imaging neonates in the NICU: initial feasibility study. Pediatr Radiol. 2012;42(11):1347-1356. doi:10.1007/s00247-012-2444-9
Figures