0662
Lung nodule imaging using high-performance 0.55T MRI1Pulmonary Branch, Division of Intramural Research, National Heart, Lung and Blood Institute, National Institutes of Health, Bethesda, MD, United States, 2Cardiovascular Branch, Division of Intramural Research, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD, United States, 3Radiology and Imaging Sciences, Clinical Center, National Institutes of Health, Bethesda, MD, United States, 4National Cancer Institute, National Institutes of Health, Bethesda, MD, United States
Synopsis
Lung imaging plays an important role in routine screening for lung nodules and lung cancer. Magnetic resonance imaging (MRI) has recently emerged as an alternative to computed tomography (CT) to image lung nodules. Lower field MRI offers high image quality, due to improved field homogeneity, and lower cost. We imaged 11 patients who had a total of 29 nodules visible on clinically motivated CT scans. T2-weighted images showed good agreement with CT in terms of nodule size and diameters and malignant nodules were more often visible on diffusion weighted images (b = 800).
Introduction
Lung imaging plays an important role in routine screening for lung nodules and lung cancer, but malignant etiology of lung nodules is difficult to rule out non-invasively. Computed tomography (CT) imaging is routinely used to image lung nodules.1 Although CT has excellent spatial resolution, it requires ionizing radiation and has limited soft tissue contrast. Magnetic resonance imaging (MRI) is a potential viable alternative to CT, offering tissue characterization and sufficient spatial resolution to detect nodules of clinically relevant sizes.1,2 Diffusion weighted MRI has previously demonstrated value in assessing nodule malignancy.3,4 A superconducting low field (<1T) MRI system has improved field homogeneity which reduces susceptibility gradients at air-tissue interfaces in the lungs. We sought to evaluate the performance of a prototype 0.55T MRI system to detect and characterize lung nodules, compared with CT and clinical diagnosis or histology.Methods
Imaging was approved by the local Institutional Review Board and all patients provided written informed consent. We imaged 11 patients with previously known lung nodules and recent clinical CT scans using a prototype 0.55T MRI system (prototype MAGNETOM Aera, Siemens Healthcare, Erlangen, Germany).5 Our MRI protocol included respiratory navigator-gated T2-weighted turbo spin echo (TSE) fast-BLADE (fBLADE) for morphological imaging (typical parameters: TR: 2800 ms, TE: 48 ms, in-plane resolution: 1.2x1.2 mm, slice thickness: 6mm, slice gap: 1.2 mm, BLADE coverage 175%, # of blades: 49, typical acquisition time: ~10 minutes) and free-breathing diffusion weighted imaging (DWI) for tissue characterization (typical parameters: TR: 5600 ms, TE: 49 ms, b-values: 50, 250, 800, (averages: 2, 4, 16), in-plane resolution: 3x3 mm, slice thickness: 6mm, slice gap: 1.2 mm, partial Fourier: 6/8, parallel imaging factor (GRAPPA): 2, acquisition time: ~9 minutes). Apparent diffusion coefficient (ADC) maps were generated from DWI images.The contemporaneous clinical CT was used to identify all nodules >4 mm. The nodule diameter and volume were measured in T2-fBLADE MRI images and compared with CT. All nodules were reported as visible or not visible in T2-fBLADE and in high b-value DWI images (b = 800), and ADC was measured in an ROI. Nodules that were stable across serial CT scans with no clinical suspicion of malignancy were considered benign. Nodules with confirmatory histology and new or growing nodules in patients with known malignancy were considered malignant.
Results
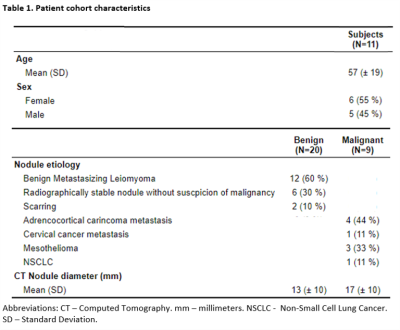
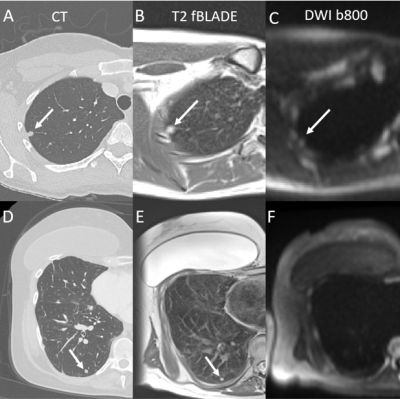
Across all 11 patients, there was a median of 0 days between CT and MRI (inter-quartile range: 0-3 days, full range: -1 - 72 days). There were a total of 29 nodules in these patients, of which 20 were benign and 9 were malignant, with diagnoses and mean diameters listed in Table 1.Example CT, T2-fBLADE and DWI images are provided in Figure 1. Figure 1A-C demonstrates a malignant nodule (8 mm CT diameter) visible on both T2-fBLADE and DWI (b = 800) images. In contrast, Figure 1D-F shows a benign small nodule (6 mm CT diameter) that was visible on T2-fBLADE and CT but not DWI (b = 800) images.
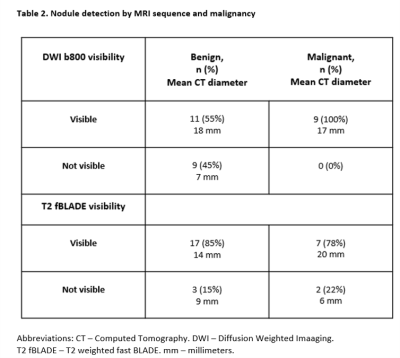
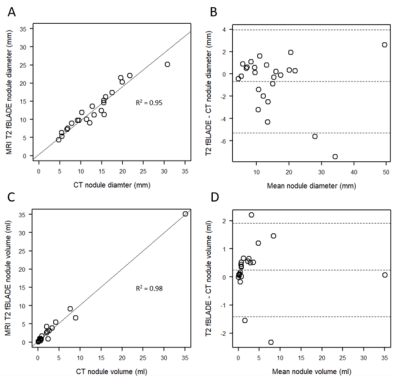
In T2-fBLADE images, 24 of 29 (83%) nodules were visible. Visibility was similar between benign and malignant nodules (Table 2). Nodule dimensions correlated well between T2-fBLADE and CT measurements, using diameter R2= 0.95 (Figure 2A) and volume R2 = 0.98 (Figure 2C) measurements. Variability was largest for smaller nodules (figures 2B and 2D). Bland-Altman analysis demonstrated a small underestimation of nodule diameter by MRI (bias = -0.7 mm), and a small overestimation of nodule volume by MRI (bias = 0.24 ml).
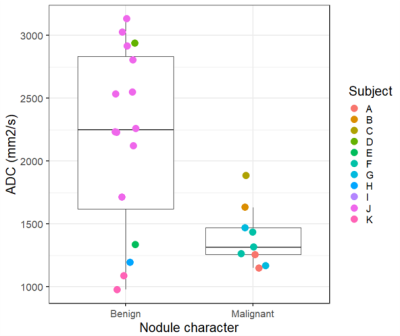
All malignant nodules were visible in high b-value (b = 800) DWI images (Table 2) but only 11 of 20 (55%) of benign nodules were visible. Median ADC was higher in benign nodules compared to malignant nodules (Figure 3), although this was driven by a single patient with n=12 nodules who had benign metastasizing leiomyoma.
Discussion
We demonstrate the potential of a high-performance 0.55T MRI system to detect malignant and benign nodules compared with CT. We used a combination of morphological imaging and tissue characterization by DWI, each with an imaging time of ~10 min. Nodule dimensions measured in T2-fBLADE images correlated strongly with CT, in agreement with previous findings.6 T2-fBLADE provided excellent tissue contrast in the lungs, but is limited by the respiratory navigator requiring end-expiration imaging. Expiratory phase images show compressed lungs which may be more prone to atelectasis and therefore higher risk of false positive nodule findings, in contrast to CT images acquired in peak inspiration. Future work is required to increase through-plane resolution for T2-weighted morphological imaging. DWI suggested that high b-value imaging at 0.55T may be a useful marker of malignancy. In our study, ADC was lower in malignant nodules although this finding was driven by a single patient with multiple nodules. Prior studies have shown ADC to be a useful marker of malignancy, although with a wide range of ADC among benign nodules.3,4Conclusions
Our results indicate that nodule screening and characterization using a high-performance 0.55T MRI system is feasible. High b-value DWI at 0.55T is a promising marker of malignancy in previously known nodules.Acknowledgements
The authors would like to acknowledge the assistance of Siemens Healthcare in the modification of the MRI system for operation at 0.55T under an existing cooperative research agreement (CRADA) between NHLBI and Siemens Healthcare. We thank Waqas Majeed and Pedro Itriago-Leon at Siemens Healthcare for assistance with the T2-fBLADE protocol. The study was supported by the Intramural Research Program, NIH/NHLBI (Z01-HL006257).References
1. Kim TJ, Kim CH, Lee HY, et al. Management of incidental pulmonary nodules: current strategies and future perspectives. Expert Rev Respir Med. 2020;14(2):173-194.
2. Meier-Schroers M, Homsi R, Schild HH, Thomas D. Lung cancer screening with MRI: characterization of nodules with different non-enhanced MRI sequences. Acta Radiol. 2019;60(2):168-176.
3. Feng H, Shi G, Liu H, Xu Q, Zhang N, Kuang J. Free-breathing radial volumetric interpolated breath-hold examination sequence and dynamic contrast-enhanced MRI combined with diffusion-weighted imaging for assessment of solitary pulmonary nodules. Magn Reson Imaging. 2021;75:100-106.
4. Koo CW, Lu A, Takahashi EA, et al. Can MRI contribute to pulmonary nodule analysis? J Magn Reson Imaging. 2019;49(7):e256-e264.
5. Campbell-Washburn AE, Ramasawmy R, Restivo MC, et al. Opportunities in Interventional and Diagnostic Imaging by Using High-Performance Low-Field-Strength MRI. Radiology. 2019;293(2):384-393.
6. Campbell-Washburn AE, Malayeri AA, Jones EC, et al. T2-weighted Lung Imaging Using a 0.55-T MRI System. Radiol Cardiothorac Imaging. 2021;3(3):e200611.
Figures