0660
Functional 3D-UTE MRI of the Lungs for Monitoring Ventilation Inhomogeneity in Patients with Cystic Fibrosis on Treatment with CFTR Modulators1Department of Diagnostic and Interventional Radiology, University Hospital Würzburg, Würzburg, Germany, 2Siemens Healthineers GmbH, Erlangen, Germany, 3Department of Pediatrics, University Hospital Würzburg, Würzburg, Germany
Synopsis
Single breath-hold 3D-UTE-MRI was proposed for simultaneous evaluation of lung morphology and function. Sixteen patients with cystic fibrosis (CF) underwent initial and follow-up 3D-UTE-MRI in 2019 and 2021. In line with a previous study, referenced voxelwise fractional ventilation (FV) provided a marker that correlates strongly with the lung clearance index (LCI), reflecting ventilation inhomogeneity. Seven patients received a new drug-combination (ivacaftor/elexacaftor/tezacaftor) with significantly improved lung function during clinical follow-up. Similarly, significantly decreased ventilation inhomogeneity was derived from functional MRI. Thus, single breath-hold 3D-UTE MRI is a valuable tool for monitoring ventilation inhomogeneity in patients with CF during drug treatment.
Introduction:
Proton-based non-contrast-enhanced lung MRI has been proven to be a viable approach for functional imaging of fractional ventilation(1–3). MRI sequences with ultrashort-echo-times (UTE) and 3D stack-of-spirals trajectories enable accelerated image acquisition within single breath-holds, achieving high signal-to-noise ratios within lung parenchyma (4,5). Amongst other free-breathing approaches, the sequential acquisition of multiple single breath-hold images with different breathing depths can be used for analysis of fractional ventilation and ventilation inhomogeneity (6,7). In cystic fibrosis (CF) a mutation of the CF transmembrane conductance regulator gene (CFTR) leads to defective CFTR-channels, leading to mucus accumulation in the airways and progressive decline of lung function. With the drug-combination of CFTR-potentiator ivacaftor, and the CFTR-correctors tezacaftor and the newly licensed compound elexacaftor a novel break-through therapy has been approved for CF-patients with at least one F508del mutation (8). The purpose of this study was to determine the ventilation inhomogeneity in patients with CF using 3D-UTE functional lung MRI and to evaluate the effect of ivacaftor/elexacaftor/tezacaftor in comparison with pulmonary function tests (PFT) and nitrogen multiple breath washout (MBW).Methods:
3D-UTE MRI imaging of the lungs was performed on a 3T MR-scanner (MAGNETOM Prisma, Siemens Healthcare GmbH, Erlangen, Germany) using a prototypical 3D stack-of-spirals sequence with a dual-density trajectory, iPAT factor 2 and SPIRiT reconstruction using the following parameters: TE = 0.05 ms; TR = 2.35 ms; flip angle = 4°, non-selective hard pulse duration = 60 µs; FOV = 600 mm x 600 mm; spiral interleaves = 264; readouts per spiral = 265; resolution = 2.3 mm isotrope; number of partitions = 102 ± 10; acquisition time per measurement = 12.3 ± 1.5 s. Sixteen patients with CF underwent MR imaging in 2019 and a follow-up examination in 2021. Six sequential single breath-hold measurements were acquired alternatedly in in- and expiration breath-holds. Images were co-registered and segmented using a deep-learning approach (9). Signal intensities were analyzed per voxel. A rational fit was performed on the data of the six measurements to correct for noise and registration uncertainties. Voxelwise fractional ventilation, expansion rate and interquartile range (IQR) of ventilation as a measurement of ventilation inhomogeneity were obtained as proposed earlier. PFT and nitrogen MBW were performed the same day. The IQR of MRI ventilation was correlated with the results of PFT and lung clearance index (LCI) from MBW tests. Data was compared between groups.Results:
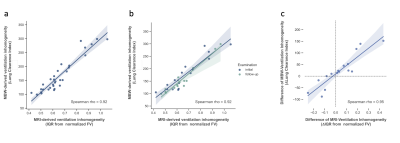
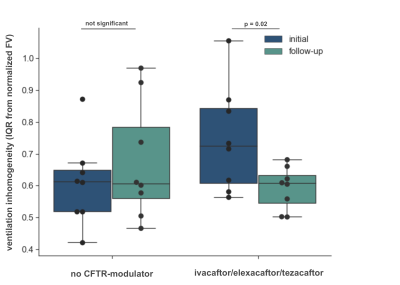
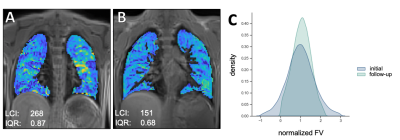
Between the initial and follow-up examination 7 patients received a new therapy with ivacaftor/elexacaftor/tezacaftor, 1 patient received another drug regimen (lumacaftor/ivacaftor). 8 patients did not receive drug therapy between both examinations. In all patients the ventilation inhomogeneity (IQR) correlated almost perfectly with the LCI2.5% in the initial and follow-up examination (Spearman’s ρ = 0.90, p < 0.01; Figure 1a). When comparing the initial and follow-up examination, comparable changes could be observed in LCI and ventilation inhomogeneity from MRI in all but one patient, shown as ΔIQR/ΔLCI (Figure 1c). In all patients who received a new therapy an improved clinical presentation was observed. In line with this, a significantly decreased IQR was obtained from functional MRI in patients on therapy (Wilcoxon p = 0.02, Figure 2), which was in line with the clinical presentation of these patients. In comparison, a slightly but not significantly elevated IQR was observed in patients without drug therapy. Figure 3 shows representative color-coded ventilation maps (a, b) and corresponding histograms of one patient who significantly benefited from therapy.Discussion:
Functional lung imaging with single breath-hold 3D-UTE MRI has been proposed as a valuable method for simultaneous qualitative and quantitative assessment of ventilation and morphology. Using a previously published approach for normalization of ventilation and calculating the distribution width yields a parameter reflecting the ventilation inhomogeneity. In a small cohort of 16 patients with cystic fibrosis a repeated examination in 2019 and 2021 demonstrates an almost perfect correlation of the MRI-derived IQR and the lung clearance index. In 8 patients who clinically benefited from a recently approved drug-combination ivacaftor/elexacaftor/tezacaftor after the initial MRI in 2019 a significantly decreased IQR was derived from functional lung MRI, which could be confirmed by a significantly improved LCI.Conclusion:
Single breath-hold 3D-UTE MRI can be a valuable tool for non-contrast-enhanced functional lung imaging for simultaneous evaluation of lung morphology and ventilation inhomogeneity during disease and drug management in patients with cystic fibrosis.Acknowledgements
No acknowledgement found.References
1. Zapke M, Topf H-G, Zenker M, et al. Magnetic resonance lung function – a breakthrough for lung imaging and functional assessment? A phantom study and clinical trial. Respir. Res. 2006;7:106 doi: 10.1186/1465-9921-7-106.
2. Kaireit TF, Sorrentino SA, Renne J, et al. Functional lung MRI for regional monitoring of patients with cystic fibrosis Latzin P, editor. PLoS One 2017;12:e0187483 doi: 10.1371/journal.pone.0187483.
3. Veldhoen S, Weng AM, Knapp J, et al. Self-gated Non–Contrast-enhanced Functional Lung MR Imaging for Quantitative Ventilation Assessment in Patients with Cystic Fibrosis. Radiology 2017;283:242–251 doi: 10.1148/radiol.2016160355.
4. Mugler JP, Meyer CH, Pfeuffer J, Stemmer A, Kiefer B. Accelerated Stack-of-Spirals Breath-hold UTE Lung Imaging. Proc. Intl. Soc. Mag. Reson. Med. 2017;p. 4904.
5. Meyer CH, Zhao L, Lustig M, et al. Dual-Density and Parallel Spiral ASL for Motion Artifact Reduction. Proc. Intl. Soc. Mag. Reson. Med 2011;64:3986.
6. Heidenreich JF, Weng AM, Metz C, et al. Three-dimensional ultrashort echo time mri for functional lung imaging in cystic fibrosis. Radiology 2020;296 doi: 10.1148/radiol.2020192251.
7. Heidenreich JF, Veldhoen S, Metz C, et al. Functional MRI of the Lungs Using Single Breath-Hold and Self-Navigated Ultrashort Echo Time Sequences. Radiol. Cardiothorac. Imaging 2020 doi: 10.1148/ryct.2020190162.
8. Bear CE. A Therapy for Most with Cystic Fibrosis. Cell 2020;180:211 doi: 10.1016/j.cell.2019.12.032.
9. Weng AM, Kestler C, Kunz AS, et al. Deep Learning Assisted Fully Automatic Post-Processing for Quantitative Lung MRI. In: Proc. Intl. Soc. Mag. Reson. Med. 27. ; 2019. p. 1720.
Figures