0651
In-vivo observations on structural aging of the inner ear – a two-center study1Institut for Neuroradiology, LMU Munich, Munich, Germany, 2Center for Vertigo and Balance Disorders, LMU Munich, Munich, Germany, 3Translational Neurosciences, University of Antwerp, Antwerpen, Belgium, 4Department of Neurology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, United States, 5Department of Otorhinolaryngology-Head & Neck Surgery, University of Antwerp, Antwerpen, Belgium, 6Institute for Neuroradiology, LMU Munich, Munich, Germany
Synopsis
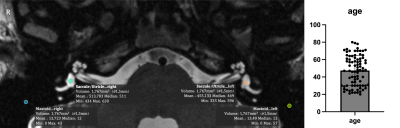
We are on the road to further the role of high-resolution structural neuroimaging in the diagnostics of the most prevalent vestibular disorders. After creating an in-vivo template and atlas space for the inner ear, we have no investigated structural aging across all cochlear and vestibular anatomical regions in a representative cohort (n=87). We significant reduction along the aging process for cochlear width and length as well as some semicircular canal dimensions. Total intracranial volume was a highly relevant covariate in our analysis. Aging also seemed to affect most neuroimaging quality parameters as well and had to be controlled for.
Introduction
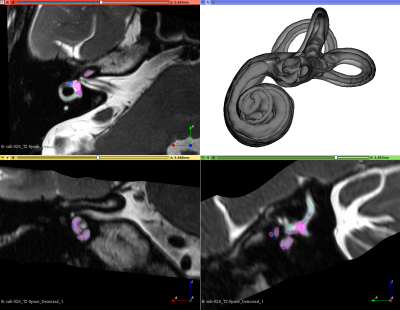
Neuroimaging of the inner ear has contributed little to diagnostics of the most prevalent vertigo disorders in clinical neurotology to date. Electrophysiology and clinical examination still dominate the puzzle solving of dizziness diseases where neuroimaging could certainly provide additional information. The lack of a dedicated human in-vivo template of the labyrinth, non-standardized high-resolution data acquisition and the absence of validated quantifiable anatomical parameters for the inner ear was identified by our group as roadblocks to increase the role of neuroimaging in clinical neurootology. We have tackled all these three objectives recently and provided the community with an in-vivo template and atlas of the human inner ear at 0.2mm isotropic resolution which has been validated against micro-CT data (Ahmadi, Raiser et al. 2021).Objective
The purpose of this study now was to investigate structural aging of the inner ear in healthy humans and at the same time test the deployment of standardized MRI sequences for the labyrinth to another center.Methods
We acquired multi-modal, isotropic MRI data (T2- and T1-weighted at 0.75mm, FLAIR at 1mm, CISS and T2-Space of the labyrinth at 0.4mm) from 87 healthy subjects (45 females, 42 males) at two sites (Munich Germany: 70, Antwerp Belgium: 17) age 20-80 years old (mean age 48.3 years) in a dedicated 3T neuroscanner (Siemens Prisma, Erlangen, Germany) with either a 64ch head/neck coil (Munich) or a 32ch head coil (Antwerp) from 2019 to 2021. Demographic and lifestyle parameters were obtained via questionnaires (e.g. SF-36 for quality of life, dizziness handicap inventory). All subjects were tested clinically for neuro-otological health upon inclusion.Data quality assessment for the T1w and T2W images was performed with MRIQC and Osirix MD software (Rosset, Spadola et al. 2004, Esteban, Birman et al. 2017). The CAT12 toolbox (http://www.neuro.uni-jena.de/cat/) (version 1742) was used to denoise and bias-field correct all neuroimaging data as well as to determine total intracranial, grey and white matter volumes as covariates of interest by segmenting the T1w images. The lesion segmentation toolbox (LST) was used to calculate the burden of white matter hyperintensities for the entire cohort from the combined FLAIR and T1w images (Schmidt, Gaser et al. 2012). Inner ear regions from the CISS and T2-Space sequences were centered, cropped (4×4×3 cm) and horizontally flipped (left inner ears to right orientation) onto our template and atlas for individual segmentation of all published anatomical subregions in an automated procedure with a Docker image incorporating deformable image-based registration after initial preregistration via 3D-Slicer (www.slicer.org)(Fedorov, Beichel et al. 2012). Statistical testing of all delineated parameters was then performed using SPSS Statistics for Macintosh Version 25 with a threshold of p<0.05.Results
Data quality analysis led to the exclusion of three subjects. Age had a significant effect on several neuroimaging quality parameters in our study including signal-to-noise-ratio of the CISS and T2-Space sequences of the inner ear (Pearson correlation for both parameters p<0.001). Total intracranial volume showed a significant positive correlation with the radius of the anterior, lateral and posterior semicircular canals as well as cochlear length (r=0.449, p=0.000) and width (r=0.523, p=0.000) in each ear. Body mass index of the subjects did not have an effect on the parameters of the inner ear fluid spaces. When controlling for total intracranial volume we found significant negative partial correlations of the anterior semicircular canal height (left r=-0.229, p=0.039; right r=-0.215, p=0.05) and common crus width (left r=-0.229, p=0.039; right r=-0.193, p=0.08) as well as cochlear length or width (left r=-0.215, p=0.051; right r=-0.221, p=0.045) with age in both inner ears.A collateral methodological finding was the significant difference for the contrast-to-noise ratio of the T1w (p=0.000) and T2w (p=0.047) images, the signal-to-noise ratio (SNR)(p=0.023) of the inner ear T2-Space sequence for the 32-channel head vs the 64-channel head/neck coil between the study sites. SNR dropped by 41% for the T2-Space sequence when using the 32ch head coil. The sequence that was most relevant for the inner ear dimensions though (CISS) was not significantly affected by the two coils with respect to SNR (64ch: SNR 27.4, 32ch: SNR 21.1).Conclusions
We found signs of structural aging (shrinkage) for dimensions of the cochlea (width and length), the common crus of the lateral and posterior semicircular canals and the height of the anterior semicircular canal. We found a significant interaction of age and neuroimaging quality parameters of the labyrinth and the whole brain alike. The previously created template and atlas provided a stable high-resolution, low-noise basis for this in-vivo MRI study on the inner ear. Structural aging of the inner ear seems to be relevant for the cochlea and few aspects of the semicircular canals, but not the main vestibulum. Thus we now have a robust data base and protocol at hand to investigate peripheral vestibular disorders in a quantifiable cross-sectional and longitudinal manner with non-invasive structural neuroimaging.Acknowledgements
This work was supported by the Federal Ministry of Education and Research (BMBF 01 EO 0901).References
Ahmadi, S. A., T. M. Raiser, R. M. Ruhl, V. L. Flanagin and P. Zu Eulenburg (2021). "IE-Map: a novel in-vivo atlas and template of the human inner ear." Sci Rep 11(1): 3293.
Esteban, O., D. Birman, M. Schaer, O. O. Koyejo, R. A. Poldrack and K. J. Gorgolewski (2017). "MRIQC: Advancing the automatic prediction of image quality in MRI from unseen sites." PLoS One 12(9): e0184661.
Fedorov, A., R. Beichel, J. Kalpathy-Cramer, J. Finet, J. C. Fillion-Robin, S. Pujol, C. Bauer, D. Jennings, F. Fennessy, M. Sonka, J. Buatti, S. Aylward, J. V. Miller, S. Pieper and R. Kikinis (2012). "3D Slicer as an image computing platform for the Quantitative Imaging Network." Magn Reson Imaging 30(9): 1323-1341.
Rosset, A., L. Spadola and O. Ratib (2004). "OsiriX: an open-source software for navigating in multidimensional DICOM images." J Digit Imaging 17(3): 205-216.
Schmidt, P., C. Gaser, M. Arsic, D. Buck, A. Forschler, A. Berthele, M. Hoshi, R. Ilg, V. J. Schmid, C. Zimmer, B. Hemmer and M. Muhlau (2012). "An automated tool for detection of FLAIR-hyperintense white-matter lesions in Multiple Sclerosis." Neuroimage 59(4): 3774-3783.
Figures