0649
In-Vivo Probabilistic Tractography of the Extracranial Branches of the Trigeminal Nerve
Kellen Mulford1, Sean Moen2, Andrew W. Grande2, David Darrow2, Donald R. Nixdorf3, Pierre-Francois Van de Moortele1, and Can Özütemiz4
1Center for Magnetic Resonance Research, University of Minnesota, Minneapolis, MN, United States, 2Department of Neurosurgery, University of Minnesota, Minneapolis, MN, United States, 3Division of TMD & Orofacial Pain, University of Minnesota, Minneapolis, MN, United States, 4Department of Radiology, University of Minnesota, Minneapolis, MN, United States
1Center for Magnetic Resonance Research, University of Minnesota, Minneapolis, MN, United States, 2Department of Neurosurgery, University of Minnesota, Minneapolis, MN, United States, 3Division of TMD & Orofacial Pain, University of Minnesota, Minneapolis, MN, United States, 4Department of Radiology, University of Minnesota, Minneapolis, MN, United States
Synopsis
Diffusion tensor imaging-based tractography of the peripheral nerves of the face is difficult owing to the complex tissue interfaces which degrade signal and introduce artifact. In this study, we used readout segmented EPI to attempt nerve fiber tracking of the inferior-alveolar, lingual, and infra-orbital branches of the trigeminal nerve. In 6 healthy participants we were able to visualize all three nerves on both sides of the face. This technique could be applies to nerve-injury and neuropathic pain populations to assess for alterations in nerve structure.
Introduction
Diffusion tensor imaging (DTI) and related tractography is a well established method of analyzing brain tissue and even adjacent portions of the peripheral nerves(1,2). The trigeminal nerve (TN) is a natural target for tractography due to its size and clinical implications in trigeminal neuralgia and nerve injury. Past research has predominantly focused on the intracranial cisternal component of the TN and typically does not extend to the extracranial sections. Tractography outside of the cranial vault is hampered by complicated tissue interfaces that decrease signal strength and introduce artifact (3, 4). This work explores the possibility of performing extracranial tractography in the largest and the most frequently injured peripheral branches of TN.Methods
MRI: Anatomic and diffusion MRI data was acquired from 6 healthy adults on a 3-Tesla MRI system using a 64-channel head and neck coil. An anatomic 3D T2-STIR sequence without contrast (TR/TE = 3/0.251 ms, FA = 120, Resolution = 0.5x0.5x0.8-mm, time = 13 minutes) was used to visualize the TN facial branches. DTI data was acquired with the RESOLVE sequence (TR/TE = 9.58/0.057 ms, FA = 180, resolution = 1.5-mm isotropic, diffusion directions = 30 , and 1 non-zero b-value of 950 s/mm2, time = 18 minutes). The DTI data was acquired twice with opposing phase encoding directions along the anterior-posterior axis.Processing: The anatomic T2-STIR volume was registered to the diffusion data without resampling. The diffusion data was preprocessed with the following steps. First, Marcenko-Pastur principal component analysis denoising was performed. Then, the anterior-posterior and posterior-anterior diffusion volumes were concatenated. Finally, the FSL scripts “topup” was used to correct for phase-encoding distortions and “eddy” was used to reduce eddy current and motion artifacts. The distribution of fiber orientations was estimated using bedpostx, and tractography was performed with “probtrackx2”, all using the FSL software package (5).
Tractography: The coregistered STIR volume was used to place all tractography seeds and waypoints in the space of the diffusion data. The initial seed was defined as the area along the cisternal segment of TN, stretching anteriorly from the pons to approximately the petrous ridge. Tractography was performed using this seed alone to define the initial spread of the intracranial cisternal segment of TN. Then, tractography was performed for each of the following nerves with the waypoint defined as follows. Inferior-alveolar nerve: waypoint placed where the nerve enters the mandible. Lingual nerve: waypoint placed just beyond the split from the inferior alveolar nerve. Infra-orbital nerve: waypoint placed just anterior to the foramen rotundum. Sample seed and waypoints can be seen in figure 1.
Results
As seen in figure 2, the results of the tractography show that the inferior alveolar, lingual, and infra-orbital nerves were successfully tracked and visualized. This result was achieved in all participants on both sides. The tractography in the inferior alveolar nerve consistently produced the highest number of streamlines, indicating a high level of connectivity, compared to the other nerves. In this nerve, the tractography stretches to the mental nerve, where the inferior-alveolar nerve exits the mandible. The lingual nerve was traced consistently to the base of the tongue. The infra-orbital nerve was traced to the base of the orbits but consistently produced the fewest number of streamlines.Discussion
Based on our first preliminary experience in the DTI of these peripheral branches, tractography extending to the face is possible, and could be used to define regions of interest for other quantitative imaging techniques, including diffusion based metrics. The tractography signal is not necessarily hampered by nearby bony structures, as the inferior alveolar nerve spends most of its course within the mandible. However, we observed fewer tractography streamlines for nerves that curve through the bony foramina or make sharp, sudden turns. Currently, tractography relies on the anatomical expertise of the person placing the seeds and waypoints. Atlas based methods may not be appropriate here, where significant differences in skull morphometry limit the accuracy of the peripheral waypoints (6). An artificial intelligence-guided seed and waypoint placement algorithm could be more promising. The primary limitation of this study is 36 minutes of diffusion scan time to collect the diffusion data, which could be clinically prohibitive. However, in select postsurgical cases, this method could be employed. If these results can be replicated with decreased scan time in the future, clinical implementation could become more feasible.Conclusion
To our knowledge, this work is the first successful demonstration of DTI based fiber tracking of the TN from the pons to the peripheral branches. While anatomic thin section imaging of small nerves have been recently introduced, these methods may not always fully identify small peripheral nerve injuries, particularly after tooth extraction or implant placement, and DTI could play a critical complementary role in these cases (7). Moreover, little is known regarding the role of peripheral distal TN branches in the setting of trigeminal neuralgia. Future applications of this technique to patients with neuropathic pain and nerve injury may yield important insights into those conditions.Acknowledgements
This research was supported by NIBIB P41 EB027061 and P30 NS076408. Personnel performing this research were also supported by the National Institutes of Health’s National Center for Advancing Translational Sciences, grants TL1R002493 and UL1TR002494. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health’s National Center for Advancing Translational Sciences.Figures
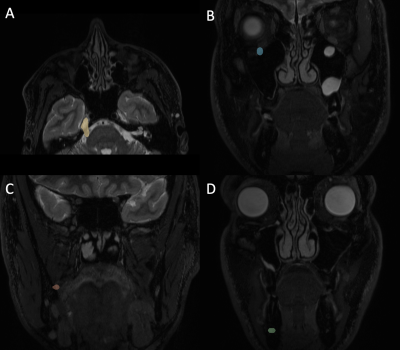
Figure 1: Sample seeds used in tractography. (A) Initial seed covering the cisternal segment of the trigeminal nerve. (B) Waypoint used in tracking the infra-orbital nerve. (C) Waypoint used in tracking the lingual nerve. (D) Waypoint used in tracking the inferior-alveolar nerve.
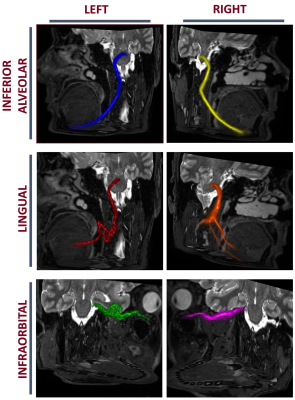
Figure 2: 3D-visualized tract-graphs results in a representative participant (Age: 36, Sex: M) overlaid on the T2-STIR volume from the same participant.
DOI: https://doi.org/10.58530/2022/0649