0629
Deuterated choline tracks choline kinase α activity and enables non-invasive assessment of response to therapy in gliomas in vivo.1Radiology, University of California San Francisco, San Francisco, CA, United States
Synopsis
Elevated choline phospholipid metabolism is a hallmark of cancer. Here, we show that silencing choline kinase a (CKα), the rate-limiting enzyme in choline phospholipid biosynthesis, abrogates total choline (tCho) production from [2H9]-choline in patient-derived glioma models, indicating that tCho production from [2H9]-choline predominantly reflects CKα activity. Importantly, we show that [2H9]-choline metabolism to tCho serves to delineate tumor from normal brain in mice bearing orthotopic patient-derived low-grade gliomas. Furthermore, [2H9]-choline informs on response to therapy, at early timepoints when anatomical alterations cannot be detected, pointing to the ability of [2H9]-choline to assess pseudoprogression, which is a challenge in glioma imaging.
Introduction
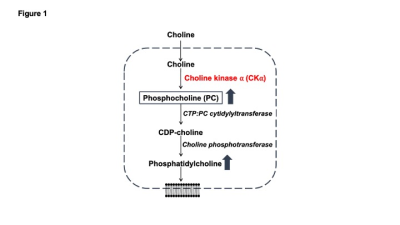
Aberrant choline phospholipid metabolism is a metabolic hallmark of cancer1,2. Choline is a dietary nutrient that is metabolized to phosphocholine (PC) by choline kinase α (CKα) and subsequently incorporated into phosphatidylcholine, which is the primary membrane phospholipid in mammalian cells1,2 (see Fig. 1). Due to the high membrane turnover associated with uncontrolled proliferation, tumor cells upregulate PC and phosphatidylcholine biosynthesis1. This metabolic phenotype has been leveraged for tumor imaging using 1H-magnetic resonance spectroscopy (MRS)-based detection of total choline (tCho; composite peak of choline, PC and glycerophosphocholine, a degradation product of phosphatidylcholine, since these peaks cannot be resolved in vivo) in several cancers including gliomas1,2. However, 1H-MRS detects steady-state metabolite levels and does not interrogate metabolic pathway activity. Recent studies suggest that 2H-MRS following administration of 2H-choline can be used to monitor production of tCho in vivo3,4. The goal of the current study was to determine whether tCho production from 2H-choline reflects CKα activity and establish the utility of 2H-choline for monitoring glioma response to therapy in vivo.Methods
Cell studies: Patient-derived high-grade glioblastoma (GBM1), low-grade oligodendroglioma (BT88) and low-grade astrocytoma (BT257) cells were maintained as previously described5-8. CKα expression was silenced by RNA interference using a mix of Smartpool siRNAs against CKα9,10. Non-targeting siRNA was used as control. Silencing was confirmed by measuring CKα (gene CHKA) mRNA levels using quantitative RT-PCR11. Cells were incubated in media containing 56mM choline chloride-(trimethyl-d9) ([2H9]-choline) for 48h. 2H-MR spectra were acquired from live cell suspensions on a Varian 14.1T scanner using a 16mm 2H surface coil and a pulse-acquire sequence (TR=260ms, NA=2500, complex points=512, flip angle=64o, spectral width=2kHz). Data was analyzed in Mnova. Peak integrals were corrected for saturation and normalized to the natural abundance of semi-heavy water (HDO, 4.75 ppm) collected from a vial containing saline.In vivo studies: We examined mice bearing orthotopic BT257 tumors generated as described earlier8. Tumor-free mice were used as healthy controls. Tumor volume was determined by T2-weighted MRI using a 14.1T scanner equipped with a single-channel 1H volume coil and a spin echo multi-slice sequence (TE=20ms, TR=1200ms, field of view=30×30 mm2, matrix=256×256, slice thickness=1mm, number of averages=4)12. For treatment response assessment, BT257 tumor-bearing mice were treated with temozolomide (50 mg/kg, 6 days/week, intraperitoneally). [2H9]-choline metabolism was monitored before (day zero, D0) and after (day 7, D7) treatment. Following injection of a bolus of [2H9]-choline (200mg/kg) via a tail-vein catheter over 2.5 min, non-localized 2H-MR spectra were acquired over 50min with a pulse-acquire sequence (TR=500ms, averages=500, complex points=512, flip angle=64, spectral width=2kHz, temporal resolution = 4min 10s). Normalized tCho levels were calculated by dividing tCho peak integrals by pre-injection HDO.
Statistical analysis: All results are expressed as mean±standard deviation. Statistical significance was assessed using an unpaired two-tailed Student’s t-test with p<0.05 considered significant.
Results
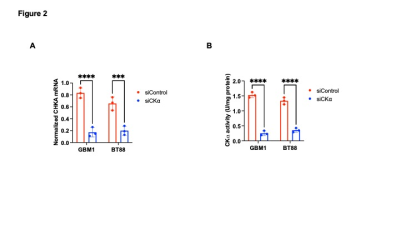
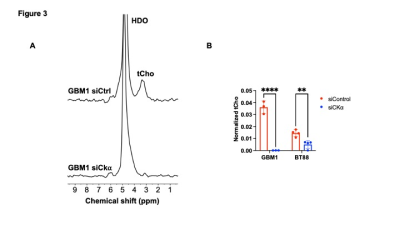
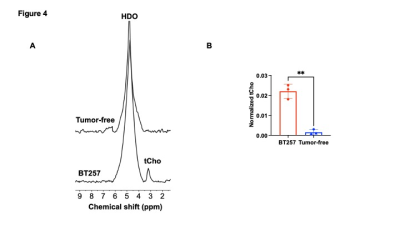
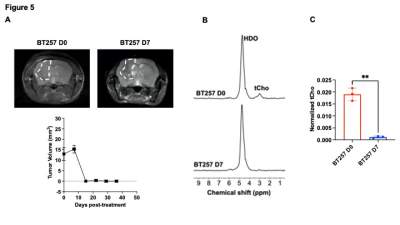
[2H9]-choline provides a readout of CKα activity: Previous studies have demonstrated tCho production in tumor regions in vivo following administration of [2H9]-choline3,4. However, since the spectral resolution of 2H-MRS does not allow differentiation of choline, PC or glycerophosphocholine, it is not clear whether the 2H-tCho peak reflects choline uptake or metabolism via CKα. We examined the effect of silencing CKα on [2H9]-choline metabolism in GBM1 and BT88 cells. First, we confirmed that CKα mRNA and activity were significantly reduced in siCKα cells relative to siCtrl (Fig. 2A-2B). Importantly, silencing CKα abrogated tCho production from [2H9]-choline in both GBM1 and BT88 models (Fig. 3A-3B). These results suggest that PC produced via CKα activity is the predominant component of the 2H-tCho peak produced from [2H9]-choline, consistent with previous studies indicating that CKα is a key rate-limiting enzyme in choline phospholipid biosynthesis in cancer1,2.[2H9]-choline can be used to non-invasively monitor response to therapy in patient-derived glioma models: Next, we examined [2H9]-choline metabolism in mice bearing orthotopic BT257 tumors and compared to tumor-free healthy controls. As shown in the representative 2H-MR spectra and quantification in Fig. 4A-4B, tCho labeling was significantly higher in BT257 tumors relative to normal brain. We then examined whether [2H9]-choline metabolism is altered by treatment with temozolomide (TMZ), which is standard of care for glioma patients13. TMZ induced tumor shrinkage as assessed by T2-weighted MRI in BT257 tumor-bearing mice, an effect that was apparent by D15 (Fig. 5A). Importantly, [2H9]-choline flux to tCho was reduced in BT257 tumor-bearing mice at D7 of TMZ treatment (Fig. 5B-5C), when no difference in tumor volume was detectable (see Fig. 5A), pointing to the potential ability of [2H9]-choline to assess early response to therapy prior to the onset of anatomical alterations.
Conclusions
To date, non-invasive methods of assessing choline phospholipid biosynthetic activity are missing. PET-based radiolabeled choline tracers interrogate choline uptake while 1H-MRS monitors steady-state choline metabolism. Our results showing that silencing CKα abrogates tCho production from [2H9]-choline in multiple patient-derived tumor models indicates that [2H9]-choline tracks the activity of CKα, which is a key rate-limiting enzyme in choline phospholipid biosynthesis. Importantly, our studies show that [2H9]-choline provides an early readout of response to therapy, prior to MRI-detectable volumetric alterations. These results suggest that [2H9]-choline has the potential to assess pseudoprogression, which is a major challenge in glioma imaging10.Acknowledgements
This study was supported by NIH R01CA239288, Department of Defense W81XWH201055315 and UCSF Brain Tumor Center Loglio and NICO initiatives.References
1 Cheng, M., Bhujwalla, Z. M. & Glunde, K. Targeting Phospholipid Metabolism in Cancer. Frontiers in oncology 6, 266, doi:10.3389/fonc.2016.00266 (2016).
2 Glunde, K., Bhujwalla, Z. M. & Ronen, S. M. Choline metabolism in malignant transformation. Nature reviews. Cancer 11, 835-848, doi:10.1038/nrc3162 (2011).
3 De Feyter, H. T., Thomas, M., Ip, K., Behar, K. & de Graaf, R. Delayed mapping of 2H-labeled choline using Deuterium Metabolic Imaging (DMI) reveals active choline metabolism in rat glioblastoma. 2021 Annual Meeting of the International Society for Magnetic Resonance in Medicine (2021).
4 Veltien, A. et al. Simultaneous Recording of the Uptake and Conversion of Glucose and Choline in Tumors by Deuterium Metabolic Imaging. Cancers (Basel) 13, doi:10.3390/cancers13164034 (2021).
5 Mancini, A. et al. Disruption of the beta1L Isoform of GABP Reverses Glioblastoma Replicative Immortality in a TERT Promoter Mutation-Dependent Manner. Cancer cell 34, 513-528 e518 (2018).
6 Giannini, C. et al. Patient tumor EGFR and PDGFRA gene amplifications retained in an invasive intracranial xenograft model of glioblastoma multiforme. Neuro-oncology 7, 164-176 (2005).
7 Kelly, J. J. et al. Oligodendroglioma cell lines containing t(1;19)(q10;p10). Neuro-oncology 12, 745-755, doi:10.1093/neuonc/noq031 (2010).
8 Mazor, T. et al. Clonal expansion and epigenetic reprogramming following deletion or amplification of mutant IDH1. Proceedings of the National Academy of Sciences of the United States of America 114, 10743-10748, doi:10.1073/pnas.1708914114 (2017).
9 Viswanath, P. et al. Mutant IDH1 gliomas downregulate phosphocholine and phosphoethanolamine synthesis in a 2-hydroxyglutarate-dependent manner. Cancer & metabolism 6, 3, doi:10.1186/s40170-018-0178-3 (2018).
10 Viswanath, P. et al. 2-hydroxyglutarate-mediated autophagy of the endoplasmic reticulum leads to an unusual downregulation of phospholipid biosynthesis in mutant IDH1 gliomas. Cancer research, doi:10.1158/0008-5472.can-17-2926 (2018).
11 Viswanath, P. et al. Non-invasive assessment of telomere maintenance mechanisms in brain tumors. Nature communications 12, 92, doi:10.1038/s41467-020-20312-y (2021).
12 Batsios, G. et al. PI3K/mTOR inhibition of IDH1 mutant glioma leads to reduced 2HG production that is associated with increased survival. Sci Rep 9, 10521, doi:10.1038/s41598-019-47021-x (2019).
13 Stupp, R. et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. The New England journal of medicine 352, 987-996, doi:10.1056/NEJMoa043330 (2005).
Figures