0617
Predictive Bladder Urodynamics Using Real-Time Magnetic Resonance Imaging: A Pilot Study1University of Wisconsin-Madison, Madison, WI, United States, 2Convergent Science, Inc., Madison, WI, United States
Synopsis
Multi-channel urodynamics is an invasive diagnostic method used to assess bladder biomechanics. MRI-based CFD has shown high potential as a clinical tool primarily focused on cardiovascular flows. In this pilot study, we demonstrate MRI-based CFD as a tool to study urodynamics. Real-time MRI on one subject provided bladder wall surfaces at multiple time points during voiding. We developed a surface mapping algorithm that processes the bladder geometries before inputting them into a CFD simulation. Coupling MRI with CFD successfully visualized and quantified urine flow dynamics. This provides a non-invasive tool to investigate urodynamics in common urological conditions such as BPH/LUTS.
Introduction
Benign prostatic hyperplasia (BPH) is the nonmalignant growth of the prostate, commonly observed in aging men. Lower urinary tract symptoms (LUTS) are the most common manifestation of BPH. Over 50% of men older than 60 years of age suffer from BPH, and 15%-30% of these men have LUTS1. Multi-channel urodynamic studies are performed to measure bladder pressure and flow during voiding, and when coupled with 2D fluoroscopy, allow visualization of urine flow. However, these techniques are invasive and do not provide sufficient information on biomechanical properties, bladder anatomy, and detrusor muscle function in BPH/LUTS2. Image-based computational fluid dynamics (CFD) has been used to study biomechanics in cardiovascular applications3,4. In this study, we expand the tools we developed for cardiovascular applications and apply them to pathological urinary flows. Here, we present a patient-specific MRI-based computational method that simulates a bladder voiding event.Methods
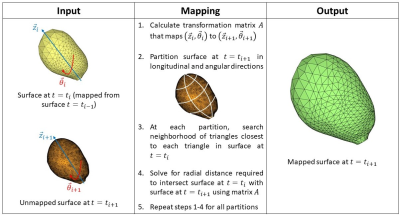
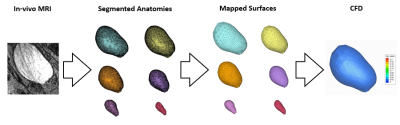
One healthy subject with no history of BPH/LUTS (29 years old) was recruited for the MRI study following an IRB-approved HIPAA-compliant protocol. The subject was scanned on a clinical 3T scanner (Premier, GE Healthcare, Waukesha, Wisconsin, USA) using 3D Differential Subsampling with Cartesian Ordering (DISCO) Flex sequence. The subject was equipped with a condom catheter prior to scanning. We acquired real-time 3D images of the bladder at multiple time phases during the voiding process. The MR images were manually segmented using a semiautomatic segmentation software Mimics and 3-matic (Materialise NV, Leuven, Belgium), providing an anatomical geometry of the bladder at each time instant. Each anatomical geometry was represented as a surface, composed of discrete triangular surface elements. We developed a novel algorithm that maps one surface to another by calculating displacements and building a transformation matrix. Figure 1 shows a schematic of the mapping algorithm. This mapping algorithm is executed between each MR time phase and provides surfaces of the bladder wall that have the same surface topology. Mapping the bladder wall surface in this manner is required for inputting the wall geometries for CFD simulation on the software package CONVERGE 3.1 (Convergent Science, Inc., Madison, Wisconsin, USA)5. CFD simulation was carried out with moving bladder wall as the driving boundary condition. Pressure at the outlet, i.e. bladder neck, was determined from a separate CFD simulation of the urethra anatomy. Wall motion was set as the transition from one mapped surface to the next, and the motion rate was determined by the time resolution of the MRI scan. Figure 2 illustrates the stages of our methods, starting from in-vivo MRI to CFD. Tecplot 360 EX (Tecplot, Inc., Bellevue, Washington, USA) was used to visualize the in-silico results.Results
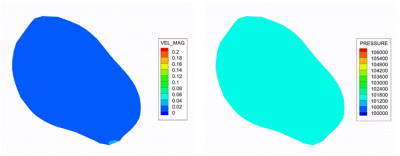
Successful image acquisition was obtained in the healthy subject where time-resolved contrast-enhanced images of the bladder during voiding were obtained in a single MRI session. The time resolution was 3.7 s and the total void time was about 74 s. After implementing the mapping algorithm, we executed a CFD simulation with MRI-based wall motion. Velocity, pressure, and wall shear stress maps of the bladder from CFD results are visualized in Figure 3. A sagittal plane was placed in the in-silico dataset and results are shown in Figure 4. CFD predicted the maximum urine flow rate (Qmax) was 12.9 cm3/s, and detrusor pressure at Qmax (PdetQmax) was 38.4 cmH20. These values were used to calculate the Bladder Outlet Obstruction Index (BOOI), and Bladder Contractility Index (BCI). CFD results show that BOOI was 12.6 (bladder not obstructed), and BCI was 92.9 (impaired bladder contractility).Discussion
Previously, bladder biomechanics, including BOOI and BCI, were measured using invasive multi-channel urodynamics. The in-vivo results only showed real-time images of the bladder at different instants but provided no information on urine flow dynamics. Coupling CFD with MRI has enabled a non-invasive method to comprehensively characterize bladder biomechanics by quantifying and visualizing urodynamics. Execution of CFD simulations is a result of the successful surface mapping algorithm we developed. The subject in this study did not present BPH/LUTS and our results show no obstruction in his bladder or urethra. However, the subject’s BCI was less than 100, indicating slightly impaired bladder contractility.Conclusion
The goal of this study was to implement a non-invasive methodology to comprehensively assess bladder biomechanics using MRI and CFD. To impose bladder motion in our CFD simulation, we developed an algorithm that maps the bladder wall surfaces (from MR images) at successive time phases. This pilot study successfully simulated, quantified, and visualized bladder voiding in one subject. Future studies would apply this procedure on multiple subjects and validate urine flow data against benchmark multi-channel urodynamics studies.Acknowledgements
The authors acknowledge support from the NIH (R01 DK126850-01).
GE Healthcare, which provides research support to the University of Wisconsin.
Convergent Science, Inc.
References
1. Thorpe A, Neal D. Benign prostatic hyperplasia. In: Lancet. Vol 361. Elsevier; 2003:1359-1367. doi:10.1016/S0140-6736(03)13073-5
2. Pewowaruk R, Rutkowski D, Hernando D, Kumapayi BB, Bushman W, Roldán-Alzate A. A pilot study of bladder voiding with real-time MRI and computational fluid dynamics. PLoS ONE. 2020;15(11 November):e0238404. doi:10.1371/journal.pone.0238404
3. Marsden AL, Feinstein JA. Computational modeling and engineering in pediatric and congenital heart disease. Current Opinion in Pediatrics. 2015;27(5):587-596. doi:10.1097/MOP.0000000000000269
4. Pewowaruk R, Roldán-Alzate A. 4D Flow MRI Estimation of Boundary Conditions for Patient Specific Cardiovascular Simulation. Annals of Biomedical Engineering. 2019;47(8):1786-1798. doi:10.1007/s10439-019-02285-2
5. Richards, K. J., Senecal, P. K., and Pomraning, E., CONVERGE 3.1, Convergent Science, Madison, WI (2021).
Figures