0614
Impacts of prenatal COVID-19 pandemic-related distress on brain functional connectivity in 3-month infants1University of Calgary, Calgary, AB, Canada, 2Department of Radiology, University of Calgary, Calgary, AB, Canada, 3Department of Pediatrics, University of Calgary, Calgary, AB, Canada
Synopsis
Prenatal psychological distress has been substantially higher during the COVID-19 pandemic, but its impacts on infant brain development remain unclear. We investigated the relationship between prenatal pandemic-related distress and functional connectivity within the default mode (DMN), auditory, and left frontoparietal brain networks in 3-month old infants. Resting-state functional MRI scans were analysed using FSL. Higher prenatal anxiety was associated with increased infant functional connectivity within the DMN. These connectivity changes may predispose these infants to later mental health challenges, and highlight the need to screen for prenatal anxiety and provide support for pregnant individuals and infants born during the pandemic.
Introduction
Typically, around 9-11% of women experience depression during pregnancy1,2, while 18-25% experience elevated anxiety symptoms3. However, rates of prenatal psychological distress have been 3-4 times higher throughout the COVID-19 pandemic in comparison to pre-pandemic rates.4,5 Depression and anxiety during pregnancy can negatively impact fetal brain function and development.6-9 Since the brain develops rapidly in utero, it is highly susceptible to exposures such as maternal distress.6,7 Mothers who experienced high levels of depression during pregnancy had infants with greater functional connectivity between the left amygdala and brain regions involved in emotion regulation.6 Similarly, children born to mothers with elevated anxiety during pregnancy had increased negative functional connectivity within the left amygdala and bilateral parietal regions of the brain7. These disruptions in functional connectivity may underlie cognitive and behavioural outcomes in children, including difficulties with emotional regulation and attention7. Therefore, we hypothesized that the pandemic-related increase in prenatal distress would have consequences on infants born during the pandemic, including changes in brain functional connectivity.Methods
Participants were recruited from the Pregnancy during the COVID-19 Pandemic (PdP)10 study. Individuals were eligible if they were pregnant, currently living in Canada, ≥17 years of age, ≥35 weeks of gestation, and able to read and write in English or French.10 Participants received repeated follow-up appointments and completed surveys during pregnancy to assess changes in mental and physical health.10 Prenatal maternal distress was measured during pregnancy using the Edinburgh Postnatal Depression Scale (EPDS)11 and the Patient Reported Outcomes Measurement Information System (PROMIS) Anxiety12 form. Infants (n=85) were recruited for resting-state functional MRI (rs-fMRI) at 3 months of age. Infants with major birth complications, or known neurological or genetic conditions were excluded from scanning. Brain imaging scans were completed using a GE 3T MR750w scanner with a 32-channel head coil at the Alberta Children’s Hospital (TR = 2000 ms, TE = 30 ms, FOV = 1920 mm, matrix = 64 x 64, voxel 3.6 x 3.6 x 3.6 mm3, flip angle = 60º, 37 slices). Participants with excessive motion (>1.5mm relative mean displacement) or those who woke up during the scan were excluded from analysis. Scans were preprocessed using FSL13 for brain extraction and motion correction. MELODIC independent component analysis (ICA) was used to remove noise components from scans, and clean scans were then registered to the 2mm Automated Anatomical Labeling (AAL) infant atlas.14 Multi-session temporal concatenation was run to create a set of common spatial components where functional networks could be identified.15 5 mm radii spheres were used to create regions of interest within the default mode (DMN), auditory, and left frontoparietal networks based on the MELODIC results (Figure 1). The average time series from these regions were extracted and correlated to quantify within-network functional connectivity. General linear models were run in SPSS to relate infant functional connectivity between each pair of regions to measures of prenatal maternal distress, with infant age, sex, maternal education, and social support included as covariates.Results
After functional imaging quality control methods were applied, 55 rs-fMRI datasets (37M/18F, 91 ± 29 days old) were retained. Higher prenatal anxiety was related to increased infant functional connectivity between anterior and posterior cingulate regions of the infant DMN (p=0.033, F=2.1, df=19) (Figure 2). No significant relationships were observed for connectivity between other pairs of regions with prenatal maternal anxiety or depression measures.Discussion
Hyperconnectivity of the DMN has previously been observed in individuals with neuropsychiatric disorders, including depression16,17, suggesting that altered brain function may predispose these infants to later mental health challenges. Prior studies have also shown that children of mothers with anxiety18 and depression19 are at higher risk of developing mental health problems themselves. Children have up to a 50% chance of developing a mental health problem when living with at least one parent with a mental illness.18 This is especially concerning during the COVID-19 pandemic since the severity and incidence of mental health concerns have increased4,5, placing pregnant individuals and their children at an even greater risk for experiencing mental health challenges. The findings of this study differ from pre-pandemic results where increased maternal anxiety was associated with reduced connectivity within default mode network-related regions20, indicating that the COVID-19 pandemic had a distinct impact on infant neurodevelopment.Conclusion
Our findings demonstrate a significant association between prenatal anxiety and functional connectivity within the infant DMN. Elevated prenatal maternal anxiety can alter infant brain development, suggesting long-term impacts of pandemic-related maternal distress on infants. Since substantial development of brain connectivity is observed in early infancy and childhood, disruptions in connectivity during these critical time periods can be detrimental to brain maturation and behaviour during infant development6,7. These results emphasize the importance of identifying and treating prenatal anxiety, and the need to support infants born during the pandemic to mitigate later negative outcomes in child neurodevelopment and mental health. Mothers with anxiety during pregnancy may also benefit from targeted interventions designed to reduce their child’s mental health risk and promote positive health outcomes.21Acknowledgements
This research was supported by the Alberta Children’s Hospital Research Institute and the Owerko Centre. G.F.G., L.T.M., and C.L. conceptualized the Pregnancy during the COVID-19 Pandemic study. A.J., K.Y.M., and C.L. had full access to all the data in the study. A.J. contributed to data collection, preprocessing, statistical analysis, interpretation, and abstract formulation. K.Y.M. contributed to data collection, preprocessing, and interpretation. K.Y.M. was supported by the T. Chen Fong Postdoctoral Fellowship in Medical Imaging Science. C.L. obtains funding from the Canada Research Chair program. All authors participated in abstract revisions and approved the final submission. The authors declare no conflicts of interest. The authors thank all families and their infants who participated in the study, scanning technicians, and members of the Developmental Neuroimaging Lab.References
2. Woody CA, Ferrari AJ, Siskind DJ, Whiteford HA, Harris MG. A systematic review and meta-regression of the prevalence and incidence of perinatal depression. J Affect Disord. 2017;219:86-92.
3. Dennis CL, Falah-Hassani K, Shiri R. Prevalence of antenatal and postnatal anxiety: systematic review and meta-analysis. Br J Psychiatry. 2017;210(5):315-323.
4. Lebel C, MacKinnon A, Bagshawe M, Tomfohr-Madsen L, Giesbrecht G. Elevated depression and anxiety symptoms among pregnant individuals during the COVID-19 pandemic. J Affect Disord. 2020;277:5-13.
5. Tomfohr-Madsen LM, Racine N, Giesbrecht GF, Lebel C, Madigan S. Depression and anxiety in pregnancy during COVID-19: A rapid review and meta-analysis. Psychiatry Res. 2021;300:113912.
6. Qiu A, Anh TT, Li Y, et al. Prenatal maternal depression alters amygdala functional connectivity in 6-month-old infants. Transl Psychiatry. 2015;5(2):e508.
7. Donnici C, Long X, Dewey D, Letourneau N, Landman B, Huo Y, Lebel C. Prenatal and postnatal maternal anxiety and amygdala structure and function in young children. Sci Rep. 2021;11(1):4019.
8. Evans J, Melotti R, Heron J, Ramchandani P, Wiles N, Murray L, Stein A. The timing of maternal depressive symptoms and child cognitive development: a longitudinal study. J Child Psychol Psychiatry. 2012;53(6):632-40.
9. Graham RM, Jiang L, McCorkle G, Bellando BJ, Sorensen ST, Glasier CM, Ramakrishnaiah RH, Rowell AC, Coker JL, Ou X. Maternal anxiety and depression during late pregnancy and newborn brain white matter development. AJNR Am J Neuroradiol. 2020;41(10):1908-1915.
10. Giesbrecht GF, Bagshawe M, van Sloten M, et al. Protocol for the pregnancy during the covid-19 pandemic (pdp) study: a longitudinal cohort study of mental health among pregnant canadians during the covid-19 pandemic and developmental outcomes in their children. JMIR Res Protoc. 2021;10(4).
11. Bergink V, Kooistra L, Lambregtse-van den Berg MP, et al. Validation of the Edinburgh Depression Scale during pregnancy. J Psychosom Res. 2011;70(4):385-389.
12. Cella D, Choi SW, Condon DM, et al. PROMIS® Adult Health Profiles: Efficient Short Form Measures of Seven Health Domains. Value Heal. 2019;22(5):537-544.
13. Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. FSL. Neuroimage. 2012;62(2):782-790.
14. Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273-89.
15. Erhardt EB, Rachakonda S, Bedrick EJ, Allen EA, Adali T, Calhoun VD. Comparison of multi-subject ICA methods for analysis of fMRI data. Hum Brain Mapp. 2011;32(12):2075-2095.
16. Greicius MD, Flores BH, Menon V, et al. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007;62(5):429-437.
17. Zhou Y, Yu C, Zheng H, Liu Y, Song M, Qin W, Li K, Jiang T. Increased neural resources recruitment in the intrinsic organization in major depression. J Affect Disord. 2010;121(3):220-30.
18. Leijdesdorff S, van Doesum K, Popma A, Klaassen R, van Amelsvoort T. Prevalence of psychopathology in children of parents with mental illness and/or addiction: an up to date narrative review. Curr Opin Psychiatry. 2017;30(4):312-317.
19. Lieb R, Isensee B, Höfler M, Pfister H, Wittchen HU. Parental major depression and the risk of depression and other mental disorders in offspring: a prospective-longitudinal community study. Arch Gen Psychiatry. 2002;59(4):365-74.
20. De Asis-Cruz J, Krishnamurthy D, Zhao L, Kapse K, Vezina G, Andescavage N, Quistorff J, Lopez C, Limperopoulos C. Association of prenatal maternal anxiety with fetal regional brain connectivity. JAMA Netw Open. 2020;3(12):e2022349.
21. Tomfohr-Madsen LM, Giesbrecht G, Madsen JW, MacKinnon A, Le Y, Doss B. Improved child mental health following brief relationship enhancement and co-parenting interventions during the transition to parenthood. Int J Environ Res Public Health. 2020;17(3):766.
Figures
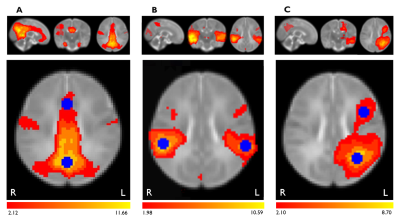
Figure 1. Within-network regions of interest used in functional connectivity analysis. Seed regions (blue) within the (A) default mode, (B) auditory, and (C) left frontoparietal networks were identified using 5 mm radii spheres. Functional connectivity (red/yellow) was quantified between pairs of seed regions in each network. Networks shown on brain maps were identified from average spatial components created using multi-session temporal concatenation in FSL.
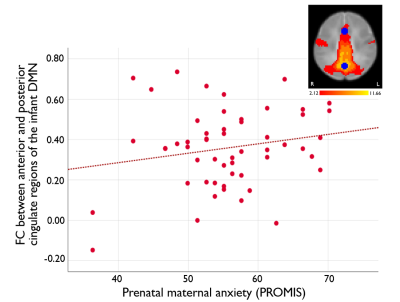
Figure 2. Association between prenatal maternal anxiety and infant default mode network (DMN) functional connectivity. Prenatal maternal anxiety, assessed using the PROMIS scores, was positively correlated with infant functional connectivity between the anterior and posterior cingulate regions of the DMN (p=0.033, F=2.1, df=19).