0611
Early Postnatal Multi-modal MRI Structural Trajectory of Brain Dysmaturation Predicts Early Childhood Language Outcomes in Complex CHD1Bioengineering, University of Pittsburgh, Pittsburgh, PA, United States, 2Radiology, UPMC Children's Hospital of Pittsburgh, Pittsburgh, PA, United States, 3Biomedical Informatics, University of Pittsburgh, Pittsburgh, PA, United States, 4Radiology, University of Pittsburgh, Pittsburgh, PA, United States, 5Epidemiology and Biostatistics, Indiana University School of Public Health-Bloomington, Bloomington, IN, United States, 6Learning and Development Center, Child Mind Institute, New York, NY, United States, 7Division of Cardiothoracic Surgery, Children’s Hospital of Philadelphia, Philadelphia, PA, United States, 8Division of Neurology, Children’s Hospital of Philadelphia, Philadelphia, PA, United States, 9Anesthesiology, Oregon Health and Sciences University, Portland, OR, United States, 10Anesthesiology, Lurie Children’s Hospital, Northwestern University, Chicago, IL, United States, 11Radiology, Texas Children's Hospital, Houston, TX, United States, 12Radiology, Texas Children's Hospital, Baylor College of Medicine, Houston, TX, United States, 13Neurology, University of Utah School of Medicine, Salt Lake City, UT, United States, 14Anesthesiology, Perioperative and Pain Medicine, Texas Children's Hospital, Baylor College of Medicine, Houston, TX, United States
Synopsis
This study examined trajectories of early postnatal brain structure (macrostructural brain volumes and microstructural white matter tractography) relationship with early childhood neurodevelopmental deficits (NDD) in complex congenital heart disease patients. This analysis included development of predictive multi-variable models incorporating other known risk factors of poor NDD in CHD. A multi-modal dysmaturation phenotype of reduced subcortical volume and cerebral white matter volume/connectivity predicted poor early childhood language outcomes, despite high relative contribution of genetic and socio-demographic factors (maternal IQ). Favorable socio-demographic factors, despite the high incidence of focal WMI and presence of genetic abnormalities, predicted better neurodevelopmental outcomes.
INTRODUCTION
Congenital heart defects (CHD), especially the severe forms – such as hypoplastic left heart syndrome (HLHS) and transposition of the great arteries (TGA) – have been shown to adversely affect neurodevelopment1,2. These neurodevelopmental deficits (NDD) include impairments in gross cognitive/intellectual functions, language/verbal abilities, and visual and motor skills in children with CHD1,3. Our primary objective was to correlate trajectories of early postnatal brain structure with early childhood NDD in complex CHD patients. Multi-modal early serial postnatal magnetic resonance imaging (MRI) – macrostructure (regional brain volumes) and microstructure fractional anisotropy (FA) of white matter (WM) tracts – was performed. Our secondary objectives were to develop predictive multi-variable models incorporating known risk factors of poor NDD in CHD.METHODS
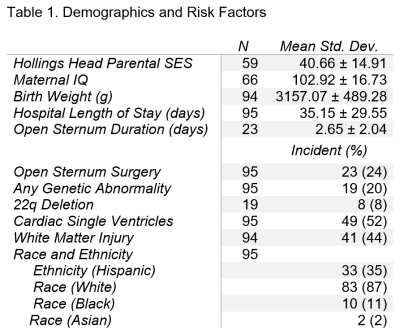
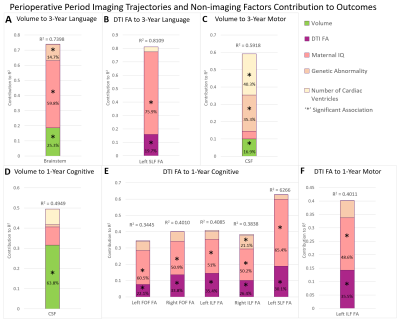
A total of 99 patients (53 male) with HLHS or TGA were prospectively recruited at a single site (Texas Children’s Hospital) and received three MRI scans – preoperatively (39.42±1.53 weeks post-conceptual age at scan), postoperatively #1 (40.66±1.53 weeks), and postoperatively #2 (61.34±7.51 weeks). All MRI scans were acquired on the same 1.5T Philips Intera scanner (Philips Medical Systems, Best, Netherlands). Each of the scans included volumetric 3D T1 imaging (TE/TR=4.0629/20ms, FOV=256mm, voxel size=1mmx1mmx1mm, flip angle=30°) and diffusion imaging (TE/TR=90/6065ms, FOV=200mm, voxel size=2mmx2mmx2.7mm, 15 gradient directions B=860s/mm2). All volumetric T1 were processed through an in-house semi-automated segmentation pipeline, Neonatal Brain Structure Segmentation4,5; and segmented into following regions: brainstem, cerebellum, cortex, intracranial cerebrospinal fluid (CSF), deep grey matter (GM), WM, whole brain (excluding CSF). Each DTI acquisition were reconstructed using diffusion tensor algorithm in DSI Studio6. Focal white matter injury (WMI) (high T1 signal) was assessed both qualitatively by neuroradiologist review (presence/absence) and continuously (volume by Licht et al). The processed DTI white matter tracts included the following: genu, body, and splenium of the corpus callosum; left and right cortico-spinal tract (CSTL and CSTR), fronto-occipital fasciculus (FOFL and FOFR), inferior longitudinal fasciculus (ILFL and ILFR), and superior longitudinal fasciculus (SLFL and SLFR). Cognitive, language, and visual/motor outcomes were assessed with the Bayley-III at 1 and 3 years. At 5 years, the full-scale IQ and verbal IQ of the WPPSI-III and the Beery VMI were used (as indices of similar outcomes to the three indices on the Bayley-III).Our three primary multi-variable models incorporated the longest epoch or overall imaging trajectory (preoperative to follow-up) predicting 5-year outcomes (n=34); 3-year outcomes (n=35); 1-year outcomes (n=45). Our secondary models incorporated two epochs of earlier trajectory imaging measures: perioperative trajectory (preoperative to postoperative) predicting 5-year outcomes (n=36); 3-year outcomes (n=35); 1-year outcomes (n=49); and post-surgical trajectory (postoperative #1 to postoperative #2) predicting 5-year outcomes (n=35); 3-year outcomes (n=37); 1-year outcomes (n=44). Single ventricle, maternal IQ and genetic abnormality were used as co-variates in the final model as they predicted a greater degree/range of ND outcome variance compared to other factors listed in Table 1. Lastly, a post-hoc factor contribution analysis was conducted for each ND test to examine the contributions of each independent variable to the variance in outcomes (Figures 1-3).
RESULTS
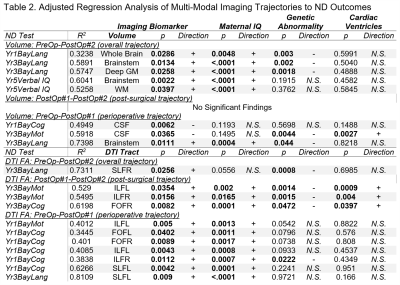
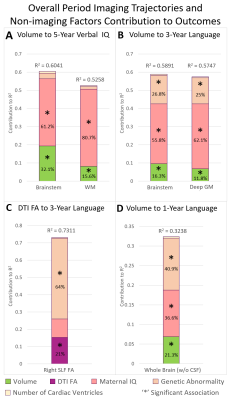
Cohort characteristics are presented in Table 1.Overall Period Multi-modal Trajectory and Early Childhood Outcome: Decreased trajectory of brainstem (p=0.0022) and WM volume (p=0.0397) predicted poor 5-year verbal IQ, while decreased trajectory of brainstem/deep GM volume and decreased trajectory of fractional anisotropy (FA) of SLFR (p=0.0256) predicted poor 3-year language outcome independent of maternal IQ/genetic abnormality.
Overall Period Multi-modal Trajectory and Infant Outcomes: Decreased trajectory of whole brain volume (p=0.0286) predicted poor 1-year language outcome independent of maternal IQ/genetic abnormality.
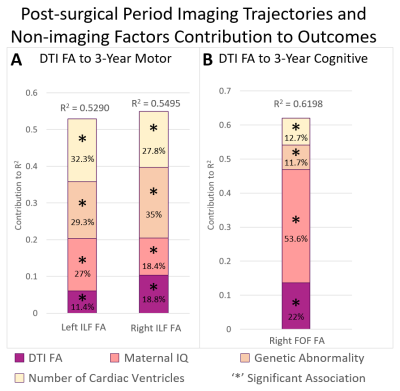
Post-surgical Microstructure (FA) Trajectory and Early Childhood Outcomes: Decreased trajectory FA of the ILFL (p=0.0354), ILFR (p=0.0156) predicted poor 3-year motor outcomes while decreased trajectory of FA of FOFR (p=0.0082) predicted poor 3-year cognitive outcomes independent of lower maternal IQ/presence of genetic abnormality/single ventricle status.
Perioperative Multi-modal Trajectory and Early Childhood Outcomes: Decreased trajectory of brainstem volume (p=0.0111) and reduced trajectory of FA of SLFL (p=0.0090) predicted poor 3-year language outcomes independent of maternal IQ.
Perioperative Multi-modal Trajectory and Infant Outcomes: Increased CSF volume (p=0.0062) and decreased trajectory of FA of ILFL (p=0.0402), ILFR (p=0.0112), FOFL (p=0.0402), FOFR (p=0.0089), SLFL (p=0.0042) predicted poor 1-year cognitive outcomes independent of maternal IQ/genetic abnormality.
DISCUSSION
Increased trajectory of CSF volume between the pre-operative and post-operative scan contributed to the greatest degree of infant ND variance (64%) relative to all imaging metrics. Post-surgical trajectories of microstructure (DTI), but not macrostructure (volume), were predictive of early childhood (3-year) cognitive and motor outcomes, suggesting vulnerability of cerebral white matter connectivity in the post-surgical period. Focal white matter injury qualitative and quantitative measures did not predict ND outcomes. A multi-modal dysmaturation phenotype of reduced subcortical volume (deep GM, brainstem) and cerebral white matter volume/connectivity predicted poor early childhood language outcomes, despite high relative contribution of genetic and socio-demographic factors (maternal IQ). Favorable socio-demographic factors (high maternal IQ, high SES), despite the presence of a high incidence of focal WMI and genetic abnormalities, were predictive of better ND outcomes suggesting the possibility of the presence of reserve mechanisms in this cohort.CONCLUSION
Reduced trajectory of multi-modal infant brain maturation (reduced subcortical volume and long-range cortico-cortical connectivity) predicts poor early childhood language outcomes independent of genetic and socio-demographic factors.Acknowledgements
Christine Johnson, Nancy H. Beluk, Jennifer Frye, Tasha Hwostow.References
- Newburger, J.W., et al., Early developmental outcome in children with hypoplastic left heart syndrome and related anomalies: the single ventricle reconstruction trial. Circulation, 2012. 125(17): p. 2081-2091.
- Bellinger, D. and J. Newburger, A Longitudinal Study From Infancy to Adolescence of the Neurodevelopmental Phenotype Associated With d-Transposition of the Great Arteries, in Congenital Heart Disease and Neurodevelopment. 2016, Elsevier. p. 27-40.
- Marelli, A., et al., Brain in congenital heart disease across the lifespan: the cumulative burden of injury. Circulation, 2016. 133(20): p. 1951-1962.
- Ceschin, R., et al., A computational framework for the detection of subcortical brain dysmaturation in neonatal MRI using 3D Convolutional Neural Networks. NeuroImage, 2018. 178: p. 183-197.
- Ceschin, R. (2021, September 20). NeBSS (Version 2021 September). Github. https://github.com/PIRCImagingTools/NeBSS
- Yeh, F. (2021, May 15). DSI Studio (Version 2021 May). Zenodo. http://doi.org/10.5281/zenodo.4764264
Figures