0610
Risk Factors for Perioperative Brain Lesions on MRI in Infants with Severe Congenital Heart Disease: A European Collaboration1Centre for the Developing Brain, School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 2Department of Neonatology, Wilhelmina Children’s Hospital, University Medical Center Utrecht and Utrecht University, Utrecht, Netherlands, 3Department of Pediatric Intensive Care, Wilhelmina Children’s Hospital, University Medical Center Utrecht and Utrecht University, Utrecht, Netherlands, 4Department of Pediatric Cardiology, Wilhelmina Children’s Hospital, University Medical Center Utrecht and Utrecht University, Utrecht, Netherlands, 5Congenital Cardiothoracic Surgery, Wilhelmina Children’s Hospital, University Medical Center Utrecht and Utrecht University, Utrecht, Netherlands, 6Utrecht Brain Center, University Medical Center Utrecht and Utrecht University, Utrecht, Netherlands, 7Child Development Center, University Children's Hospital Zurich, Zurich, Switzerland, 8Department of Pediatrics, Beatrix Children’s Hospital, University Medical Center Groningen, Groningen, Netherlands, 9Department of Obstetrics, Wilhelmina Children’s Hospital, University Medical Center Utrecht and Utrecht University, Utrecht, Netherlands, 10Pediatric Heart Center, University Hospital Giessen, Justus-Liebig-University Giessen, Giessen, Germany, 11Department of Congenital Heart Disease and Pediatric Cardiology, German Heart Center Munich, Technical University of Munich, Munich, Germany, 12Department of Diagnostic Imaging, University Children’s Hospital Zurich, Zurich, Switzerland, 13Department of Neonatology and Pediatric Intensive Care, University Children’s Hospital Zurich, Zurich, Switzerland, 14Division of Congenital Cardiovascular Surgery, Pediatric Heart Centre, University Children’s Hospital Zurich, Zurich, Switzerland, 15Paediatric Cardiology Department, Evelina London Children's Healthcare, London, United Kingdom, 16Biomedical Engineering Department, School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 17Pediatric Cardiology, Pediatric Heart Center, Department of Surgery, Children’s Research Center, University Children’s Hospital, and University of Zurich, Zurich, Switzerland
Synopsis
Infants with Congenital Heart Disease (CHD) are at risk of neurodevelopmental impairments. MRI studies have identified brain injury in infants with CHD both before and after cardiac surgery. We characterized risk factors for preoperative and new postoperative arterial ischaemic stroke (AIS), white matter injury (WMI) and cerebral sinovenous thrombosis (CSVT) in a cohort of infants with severe CHD. Balloon atrial septostomy was associated with increased risk of preoperative brain injury. Induced vaginal delivery was associated with preoperative WMI. Cardiac physiology and perioperative factors were associated with increased risk of new postoperative brain injury.
Introduction
Infants with Congenital Heart Disease (CHD) are at increased risk of neurodevelopmental impairments across multiple domains1,2. The underlying mechanisms are unclear. MRI studies have identified brain injury in infants with CHD both before and after cardiac surgery3-5. White matter injury (WMI) and arterial ischemic stroke (AIS) are the most commonly reported lesions6-9. In addition, infants with CHD may also show cerebral sinovenous thrombosis (CSVT) on perioperative MRI. Identification of modifiable risk factors is essential to reduce brain injury. Our aim was to characterize risk factors for preoperative and new postoperative AIS, WMI and CSVT in a cohort of infants with CHD requiring cardiac surgery in the first six weeks after birth.Methods
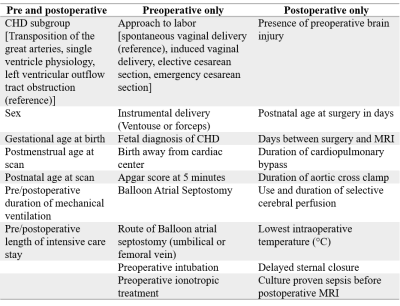
Three prospective observational cohort studies from Wilhelmina Children’s Hospital Utrecht (WKZ 2016-2019), University Children’s Hospital Zurich (UCZ 2009-2019) and St Thomas’ Hospital London (KCL 2014-2019) were combined. Infants with CHD [Median (25-75 quartile) gestational age at birth median= 39.0 (38.3-40.0) weeks] who underwent corrective or palliative cardiac surgery during the first 6 weeks after birth were included. CHD included in the study were transposition of the great arteries (TGA) (with or without arch obstruction), single ventricle physiology (SVP), or left ventricular outflow tract and/or aortic arch obstruction (LVOTO). Infants with known or suspected genetic or syndromic disorders and other types of CHD were excluded.180 infants underwent a 3T brain MRI preoperatively, 146 of which were also imaged postoperatively. T1, T2, diffusion and susceptibility-weighted images were acquired. Infants in WKZ and KCL also underwent MR venography. MRI scans from each cohort were assessed using a uniform description of brain imaging findings9. Clinical characteristics were collected prospectively at each center and subsequently combined. Postoperative brain MRI findings [Median (25-75 quartile) days from surgery= 10 (7-15)] were considered new if preoperative MRI showed no corresponding findings, lesion(s) were in a different location, and/or there was an increase in size or number compared to preoperative findings. Potential risk factors (Table 1) for presence of WMI, AIS and CSVT were assessed with univariate group differences and backward stepwise multivariate logistic regressions. Input variables for the regression were selected based on previous literature, outcomes from univariate analyses, and whether factors were modifiable.
Results
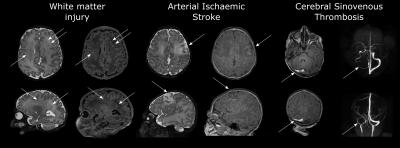
Examples of WMI, AIS and CSVT are given in Figure 1. The number of infants who had WMI, AIS and CSVT pre- and postoperatively are summarized in Table 2. Induced vaginal delivery (OR 2.23, 95% CI 1.06-4.70) was associated with preoperative WMI. Balloon atrial septostomy was associated with WMI (OR 2.51, 95% CI 1.23-5.20) and AIS preoperatively (OR 4.49, 95% CI 1.20-21.49). Absolute risk of preoperative WMI increased from 15% (95% CI 9%-24%) to 28% (95% CI 17%-43%) after induced vaginal delivery, 31% (95% CI 20%-44%) after balloon atrial septostomy and 50% (95% CI 32%-68%) in infants who underwent both. The absolute risk of preoperative AIS increased from 3% (95% CI 0.8%-8%) to 13% (95% CI 6%-24%) after undergoing balloon atrial septostomy. SVP was a risk factor for new postoperative WMI (OR 2.88 95% CI 1.20-6.95). Younger postnatal age in days at surgery (OR 1.18, 95% CI 1.05-1.33) and selective cerebral perfusion, particularly combined with deep hypothermia (≤20°C) (OR 13.46, 95% CI 3.58-67.10), were independent risk factors for new postoperative AIS. Transposition of the great arteries (OR 13.47, 95% CI 2.28-95.66) delayed sternal closure (OR 3.47 95% CI 1.08-13.06) and lower minimum intraoperative temperature (OR 1.22, 95% CI 1.07-1.36) were associated with new postoperative CSVT.Discussion
This study confirms previous findings that balloon atrial septostomy is associated with increased risk of preoperative brain injury10 and that infants with single ventricle physiology are at higher risk for postoperative WMI8,11. Our results suggest that delivery planning and timing of surgery encompass modifiable risk factors that may allow clinicians to personalize treatment to minimize the risk of perioperative brain injury in CHD. It is important to note in our centers, balloon atrial septostomy is performed in infants with unacceptably low preductal oxygen saturations. Infants who are delivered by planned induction may be exposed to adverse intrauterine conditions. In addition, both induced vaginal delivery and balloon atrial septostomy are utilized in infants who are susceptible to cardiovascular disruption postnatally. We therefore cannot be certain whether the interventions themselves or the associated indications increase susceptibility to brain injury. Further research is needed to optimize cerebral perfusion techniques for neonatal surgery and to confirm the relationship between CSVT and perioperative risk factors.Acknowledgements
This research was funded by a Consolidator Grant of the European Society of Paediatric Research (ESPR). WKZ: Funding by the Hartekind Foundation and Vrienden van het Wilhelmina Kinderziekenhuis Foundation and by ZONMW (doelmatigheidsonderzoek) Crucial trial. KCL: Funding by Action Medical Research (GN2630), the Medical Research Council UK (MR/V002465/1; MR/L011530/1) and the British Heart Foundation (FS/15/55/31649) and support from core funding from the Wellcome/EPSRC Centre for Medical Engineering [WT203148/Z/16/Z] and by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London and/or the NIHR Clinical Research Facility. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care. UCZ: Funding by the Mäxi-Foundation, the Anna Müller Grocholski Foundation and the EMDO Foundation Zurich.References
1. Marino BS, Lipkin PH, Newburger JW, et al. Neurodevelopmental outcomes in children with congenital heart disease: evaluation and management: a scientific statement from the American Heart Association. Circulation 2012;126:1143–1172.
2. Feldmann M, Bataillard C, Ehrler M, et al. Cognitive and Executive Function in Congenital Heart Disease: A Meta-analysis. Pediatrics 2021;148:e2021050875.
3. Miller SP, McQuillen PS, Hamrick S, et al. Abnormal brain development in newborns with congenital heart disease. N Engl J Med 2007;357:1928–1938.
4. Mebius MJ, Kooi EMW, Bilardo CM, et al. Brain Injury and Neurodevelopmental Outcome in Congenital Heart Disease: A Systematic Review. Pediatrics 2017;140:e20164055.
5. Kelly CJ, Arulkumaran S, Tristão Pereira C, et al. Neuroimaging findings in newborns with congenital heart disease prior to surgery: an observational study. Arch Dis Child 2019;104:1042 LP – 1048.
6. Beca J, Gunn J, Coleman L, et al. Pre-Operative Brain Injury in Newborn Infants With Transposition of the Great Arteries Occurs at Rates Similar to Other Complex Congenital Heart Disease and Is Not Related to Balloon Atrial Septostomy. J Am Coll Cardiol 2009;53:1807–1811.
7. Chen J, Zimmerman RA, Jarvik GP, Nord AS, et al. Perioperative stroke in infants undergoing open heart operations for congenital heart disease. Ann Thorac Surg 2009;88:823–829.
8. Beca J, Gunn JK, Coleman L, et al. New white matter brain injury after infant heart surgery is associated with diagnostic group and the use of circulatory arrest. Circulation 2013;127:971–979.
9. Stegeman R, Feldmann M, Claessens NHP, et al. A Uniform Description of Perioperative Brain MRI Findings in Infants with Severe Congenital Heart Disease: Results of a European Collaboration. Am J Neuroradiol 2021.
10. McQuillen PS, Hamrick SEG, Perez MJ, Barkovich AJ, Glidden D V., Karl TR, et al. Balloon Atrial Septostomy Is Associated With Preoperative Stroke in Neonates With Transposition of the Great Arteries. Circulation 2006;113:280–285.
11. Peyvandi S, Kim H, Lau J, Barkovich AJ, Campbell A, Miller S, et al. The association between cardiac physiology, acquired brain injury, and postnatal brain growth in critical congenital heart disease. J Thorac Cardiovasc Surg 2018;155:291-300.e3. doi:10.1016/j.jtcvs.2017.08.019.
Figures