0608
Measurement of pancreas graft temperature during cold preservation in MR scanner1Department of Medical Physics, Uppsala University Hospital, Uppsala, Sweden, 2Department of Immunology, Genetics and Pathology, Uppsala University, Uppsala, Sweden
Synopsis
Phosphorus (31P) and proton (1H) magnetic resonance spectroscopy (MRS) are the methods of choice in the assessment of the pancreas graft quality before organ or islet of Langerhans transplantation. During the transport and MR scanning hypothermic storage (4±2 oC) has to be maintained. The aim of this study was to investigate if it is possible to measure pancreas graft temperature in a MR scanner using 1H-MRS and temperature constants obtained by the calibration experiments with the water-vegetable oil phantom. The present study has shown that 1H-MRS is able to measure the graft temperature during MR scanning.
Introduction
Transplantation of pancreas or islets of Langerhans belong to the most effective treatments for patients suffering type 1 or 2 diabetes. However, the pancreas is regarded as one of the most challenging organs for recovery and transplantation. The objective assessment of pancreas graft quality is the key factor for success in transplantation. The aim of such evaluation methods is to predict either pancreas donor utilization or graft failure. Possible methods of choice are phosphorus (31P) 1,2 and proton (1H) magnetic resonance spectroscopy (MRS). 3,4 During the transport and MR scanning hypothermic storage (4±2 oC) and sterile conditions need to be maintained. Since MR scanning can increase the temperature of the pancreas graft, measurement of the graft temperature is desirable. The aim of this study was to investigate if it is possible to measure pancreas graft temperature in MR scanner using 1H-MRS.Methods
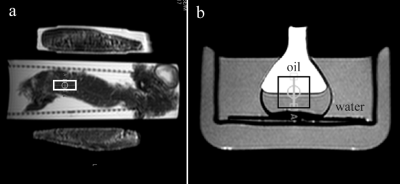
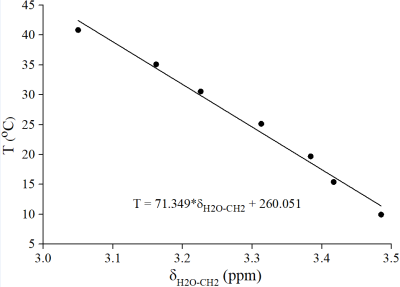
Pancreas grafts from 10 human donors (4 female) were included in this study. Median donor age and body mass index was 65.5 years (range: 24-82), and 26.8 kg/m2 (range: 22.2-31.9), respectively. Organ donation was performed solely for research purposes. The study was approved by The Regional Ethics Committee. Each pancreas was perfused in-situ with histidine-tryptophan-ketoglutarate (HTK) solution and placed into a transport container filled with HTK solution and cooled by ice packs. MR experiments were performed on a 1.5 T scanner (Philips, Achieva) using transmit-receiver head coil. Single-voxel MRS acquisitions of pancreas were performed using PRESS sequence (TR/TE 5000/30 ms, BW 1000 Hz, 1024 time domain points). Figure 1a shows voxel (10x10x25 mm3) position. Four dummy excitations were followed by 16 non-water-suppressed and 64 water-suppressed scans. The 1H-MRS temperature calibration was performed using the phantom contained vegetable oil (sunflower) and water. Phantom was heated and cooled in a thermally insulated water bath (Fig. 1b). Warming/cooling of the water bath was performed with thermostatically controlled water flowing through the walls of the glass cylinder. Seven PRESS acquisitions (TR/TE 2000/80 ms, 16 non-water-suppressed, 48 water-suppressed scans) at the thermal equilibrium were performed over a temperature range of 10-41 oC. The phantom temperature was measured with the precision ±0.1 oC using a Pt-100 probe. The linear regression analysis (Fig. 2) was performed between the temperature T and water-vegetable oil ((CH2)n spectral line) chemical shift difference δH2O-CH2. The equation of regression line was used to compute the temperature of pancreas graft. Graft was measured in its original, unopened plastic transport container. During scanning the hypothermic storage was maintained by cooling ice elements in thermally insulated box (Fig. 1a). Spectral intensities were fitted in the time domain by the AMARES/MRUI algorithm. Prior knowledge used for the fitting of the pancreas lipid signals originating from adipocytes (AD) and intracellular lipids (IC) of non-adipose pancreatic cells is described elsewhere. 5,6Results and discussion
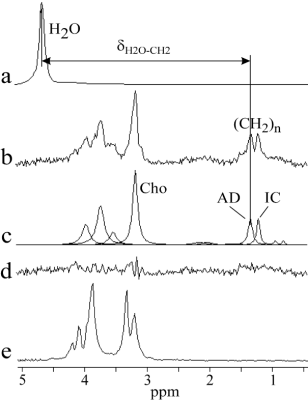
The air (pancreas graft) temperature inside the transport package was 4.6±0.7 oC (range: 3.5-5.9) at the time of delivery to our laboratory, i.e. immediately before MR scanning. It was not possible to open transport container and to measure temperature of the pancreas by conventional sensors due to strict requirement for sterility. Linear regression analysis yielded the temperature (oC) relationship: T= -71.349(±3.683)*δH2O-CH2 + 260.051(±12.133) (Fig. 2). The representative pancreas graft spectrum and spectrum of HTK solution are shown in Fig. 3. Mean water/fat (CH2)n spectral intensity ratio of the considered seven pancreas grafts was 1.5±1.6% (range: 0.2-4.7). Pancreas graft temperature was computed from the chemical shift difference between temperature depended water resonance and a temperature independent (significant less dependent) reference methylene line (CH2)n originating from the pancreatic adipocytes (AD). It should be noted that total choline (Cho) resonance at 3.2 ppm was not possible to be used as the temperature reference because of overlapping by spectral lines of HTK solution (histidine) (Fig. 3). Two spectra were discarded because of insufficient shimming results and one spectrum was excluded due to failure in fitting of (CH2)n resonance of adipocytes. Mean temperature of seven pancreas grafts was 3.6±1.3 oC (range: 2.0-5.7). This result is in line with the grafts temperature 4.6±0.7 oC (range: 3.5-5.9) at the time of delivery to our hospital, i.e. a few minutes before the MR scanning.Conclusion
We have demonstrated that the temperature of pancreas graft in MR scanner can be measured by means of 1H-MRS and by using the temperature constants obtained by temperature calibration experiments with the water-vegetable oil phantom.Acknowledgements
No acknowledgement found.References
1. Scott WE, Weegman BP, Ferrer-Fabrega J, et al. Pancreas oxygen persufflation increases ATP levels as shown by nuclear magnetic resonance. Transplant Proc 2010;42:2011-2015.
2. Carlbom L, Weis J, Johansson L, et al. Pre-transplantation 31P-magnetic resonance spectroscopy for quality assessment of human pancreatic grafts – A feasibility study. Magn Reson Imag 2017;39:98-102.
3. Tushuizen ME, Bunck MC, Pouwels PJ, et al. Pancreatic fat content and β-cell function in men with and without type 2 diabetes. Diabetes Care 2007;30:2916-2921.
4. Lingvay I, Esser V, Legendre JL, et al. Noninvasive quantification of pancreatic fat content in humans. J Clin Endocrinol Metab 2009;94:4070-4076.
5. Weis J, Johansson L, Ortiz-Nieto F, Ahlström H. Assessment of lipids in skeletal muscle by LCModel and AMARES. J Magn Reson Imag 2009;30:1124-1129.
6. Weis J, Ahlström H, Korsgren O. Proton MR spectroscopy of human pancreas allografts. MAGMA 2019;32:511-517.
Figures