0605
Free Breathing Abdominal Tissue Quantification using 2D MR Fingerprinting with quadratic RF phase (qRF-MRF)1Biomedical Engineering, Case Western Reserve University, Cleveland, OH, United States, 2Radiology, Case Western Reserve University, Cleveland, OH, United States, 3Physics, Case Western Reserve University, Cleveland, OH, United States, 4Radiology, University Hospitals Cleveland Medical Center, Cleveland, OH, United States
Synopsis
This study presents a Pilot Tone (PT) based free-breathing technique for two-dimensional simultaneous quantification of T1, T2, T2*, fat fraction (FF), and water fraction (WF) using quadratic RF phase Magnetic Resonance Fingerprinting (qRF-MRF). We report the quantitative comparison of a cohort of 10 healthy subjects between free-breathing and breath-hold qRF-MRF as well as the comparison to standard clinical protocols. Free-breathing results are comparable to both breath-hold and standard clinical protocols (p > 0.05), indicating the stability and reproducibility of the method.
Introduction
This study demonstrates a robust framework for free-breathing Pilot Tone (PT) (1–3) navigator based 2D-quadratic RF (qRF) Magnetic Resonance Fingerprinting (MRF) for inherently co-registered quantitative T1, T2, T2*, off-resonance, fat fraction (FF), and water fraction (WF) in the abdomen with ten healthy volunteers.Methods
Acquisition:This study was performed on a 3.0-T Siemens Vida scanner (Siemens Healthineers). We designed a qRF-MRF sequence (4,5) with an optimized frequency sweep, inversion efficiency, and FA pattern for improved T1, T2 and T2* sensitivity (6) while maintaining a reasonable breath-hold duration: 1758 time points; TR=8.1 msec; total scan time=15 s; FOV, 400 x 400 mm2; matrix size, 256 x 256; slice thickness, 5 mm. Standard clinical breath-hold sequences were applied at the same slice position to acquire the same quantitative information, which included 3 separate acquisitions/breath-holds: T1 MOLLI, T2 FSE, T2* map, FF map, and WF map (VIBE q-Dixon, Siemens LiverLab).
PT:
The PT navigator was implemented with retrospective gating as described in the literature (4,7). The function generator was synchronized to the 10 MHz clock of the scanner. PT signals were encoded in the raw data through receiver arrays (TIM body 18, spinal arrays, Siemens Healthineers). Principal component analysis (PCA) was applied to PT signals extracted from all coils. The navigator signal was chosen from the principal component that showed the most relative power in the frequency band of the respiratory motion. The temporal resolution of the Pilot Tone signal was determined by sequence repetition time (8.1 ms). Ten measurements of an axial slice were acquired to ensure sufficient respiratory data has been collected. In between each measurement, a wait time of ten seconds was applied to ensure full longitudinal recovery. The total acquisition time was four minutes.
Reconstruction:
The qRF-MRF dictionary was generated using the Bloch simulations with combinations of signal evolution with T1 in a range of 10 – 5000ms, T2 and T2* in a range of 2 – 2000ms, and off-resonance in a range of -62 Hz to 62 Hz. The dictionary was compressed using rSVD (8) to reduce memory requirement. Quadratic interpolation was used to improve the resolution of the dictionary (9). To improve image quality in reconstruction, iterative low-rank reconstruction was implemented for both breath-hold and free-breathing PT scans (10).
The end-inspiration and end-expiration states were defined by PT thresholds. Approximately 3000 time-points in each respiratory state were selected. The spiral sampling distribution was compensated based on the occurrence of each spiral arm. A pattern-matching algorithm was performed on the subset image series with the corresponding compressed dictionary and trajectory (11). A partial volume MRF dictionary was generated based on T1, T2, T2* values of each subject to create the FF and WF maps (12).
In Vivo Study:
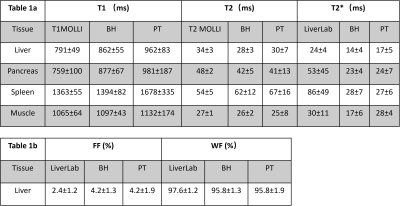
Ten healthy volunteers (five men [mean age, 23; range, 19-26 years] and five women [mean age, 27.2 years; range, 20-47 years]) were recruited in this study and written consent forms were obtained from all the subjects before the experiments. Similar slice positions using free-breathing qRF MRF, breath-hold qRF MRF, and clinical acquisitions were performed on the volunteers for visual and quantitative comparison. ROI analysis was performed to extract T1, T2, and T2* from multiple organs in the abdomen, including liver, pancreas, spleen, and muscle. ROI analysis of FF and WF was extracted from the liver.
Results
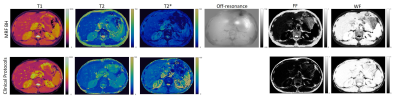
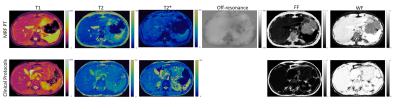
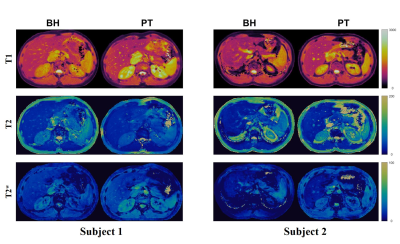
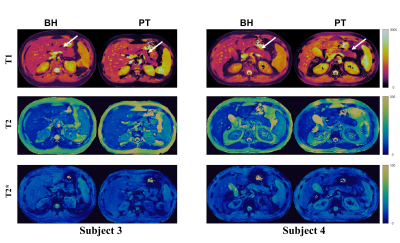
Figure 1 shows the 2D T1, T2, T2*, off-resonance, FF, and WF maps of the liver using a breath-hold qRF-MRF acquisition along with the corresponding T1 MOLLI, T2 FSE, and T2* LiverLab output. Figure 2 shows the free-breathing 2D T1, T2, T2*, off-resonance, FF, and WF maps of the liver along with the breath-hold T1 MOLLI, T2 FSE, and T2* LiverLab output of the same subject. Figure 3 compares breath-hold qRF-MRF against PT qRF-MRF of 2 subjects in the liver. Figure 4 shows breath-hold and PT qRF-MRF of 2 different subjects and pancreas-focused slice positions. Table 1 is a summary of the quantitative values acquired from all 10 subjects.Discussion and Conclusion
This work shows quantitative results of both breath-hold and free-breathing qRF-MRF from ten healthy subjects. The resulting T1, T2, T2* relaxation times indicate that free-breathing qRF-MRF results are comparable to breath-hold qRF-MRF (p > 0.05). Therefore, with the optimized qRF-MRF sequence, free-breathing maps are stable and have the same level of quality as the BH maps. Furthermore, qRF-MRF results are comparable to clinical protocols while having the benefits of simultaneously generating T1, T2, T2*, off-resonance, FF, and WF using one acquisition. As demonstrated in the clinical images shown in Figures 1 and 2, some variations in 2D slice position exist across acquisitions due to acquisitions obtained from multiple breath-holds. One advantage of qRF-MRF is that all six quantitative maps are inherently co-registered and convenient for direct comparison. Finally, free-breathing acquisition using this framework is robust and reproducible across all subjects and organs tested in this work (Figure 3,4 and table 1). This method demonstrates the feasibility of a robust framework that can simultaneously map T1, T2, T2*, off-resonance, FF, and WF to characterize diseases throughout the abdomen using a completely free-breathing acquisition.Acknowledgements
This material is supported by Siemens Healthcare and the National Science Foundation Graduate Research Fellowship Grant No. CON501692. This work was supported by the Interdisciplinary Biomedical Imaging Training Program, NIH T32EB007509 administered by the Department of Biomedical Engineering, Case Western Reserve University. This report is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.References
1. Huang SS, Boyacioglu R, Bolding R, MacAskill C, Chen Y, Griswold MA. Free-Breathing Abdominal Magnetic Resonance Fingerprinting Using a Pilot Tone Navigator. J. Magn. Reson. Imaging 2021;54:1138–1151 doi: 10.1002/jmri.27673.
2. Ludwig J, Speier P, Seifert F, Schaeffter T, Kolbitsch C. Pilot tone-based prospective respiratory motion correction for 2D cine cardiac MRI. In: Pilot tone-based prospective respiratory motion correction for 2D cine cardiac MRI. Vol. 27. Montreal: International Society For Magnetic Resonance in Medicine; 2019. p. 73. doi: 10.1007/s10334-015-0487-2.
3. Vahle T, Bacher M, Rigie D, et al. Respiratory Motion Detection and Correction for MR Using the Pilot Tone: Applications for MR and Simultaneous PET/MR Examinations. Invest. Radiol. 2020;55:153–159 doi: 10.1097/RLI.0000000000000619.
4. Huang S, Chen Y, Bolding R, Bittencourt LK, Griswold M, Boyacioglu R. Free Breathing 2D Abdominal Magnetic Resonance Fingerprinting with quadratic RF phase. In: Proc. Intl. Soc. Mag. Reson. Med. Virtual; 2021. p. 0478.
5. Boyacioglu R, Wang C, Ma D, McGivney DF, Yu X, Griswold MA. 3D magnetic resonance fingerprinting with quadratic RF phase. Magn. Reson. Med. 2020 doi: 10.1002/mrm.28581.
6. Boyacioglu R, Huang S, Chen Y, Griswold M. Dictionary variance based optimization of MRF. In: Proc. Intl. Soc. Mag. Reson. Med. Submitted; 2022.
7. Huang S, Boyacioğlu R, Bolding R, Chen Y, Griswold MA. Free-Breathing Abdominal Magnetic Resonance Fingerprinting Using a Pilot Tone Navigator. In: Proc. Intl. Soc. Mag. Reson. Med. Virtual.
8. Yang M, Ma D, Jiang Y, et al. Low rank approximation methods for MR fingerprinting with large scale dictionaries. Magn. Reson. Med. 2018;79:2392–2400 doi: https://doi.org/10.1002/mrm.26867.
9. McGivney D, Boyacioglu R, Jiang Y, Wang C, Ma D, Griswold M. Towards Continuous Dictionary Resolution in MR Fingerprinting using a Quadratic Inner Product Model. In: 25th Annual Meeting of ISMRM. Vol. 27. Montréal, QC, Canada; 2019. p. 4526.
10. Hamilton JI, Jiang Y, Ma D, et al. Simultaneous multislice cardiac magnetic resonance fingerprinting using low rank reconstruction. NMR Biomed. 2019;32:e4041 doi: https://doi.org/10.1002/nbm.4041.
11. Ma D, Gulani V, Seiberlich N, et al. Magnetic Resonance Fingerprinting. Nature 2013;495:187–192 doi: 10.1038/nature11971.
12. Deshmane A, McGivney DF, Ma D, et al. Partial volume mapping using magnetic resonance fingerprinting. NMR Biomed. 2019;32:e4082 doi: https://doi.org/10.1002/nbm.4082.
Figures