0603
Free-Breathing, 3D Phase Sensitive Inversion Recovery T1-Weighted MRI of the Liver1Department of Biomedical Engineering, University of Wisconsin-Madison, Madison, WI, United States, 2Department of Radiology, University of Wisconsin-Madison, Madison, WI, United States, 3GE Healthcare, Waukesha, WI, United States, 4GE Healthcare, Atlanta, GA, United States, 5Department of Medical Physics, University of Wisconsin-Madison, Madison, WI, United States, 6Department of Medicine, University of Wisconsin-Madison, Madison, WI, United States, 7Department of Emergency Medicine, University of Wisconsin-Madison, Madison, WI, United States
Synopsis
Gadoxetic acid (GA)-enhanced hepatobiliary phase T1-weighted (HBP-T1w)-MRI is widely used in the clinical setting for detection of focal liver lesions (FLLs). However, complete characterization of liver lesions also requires T2w, diffusion weighted (DWI) and dynamic contrast enhanced (DCE) imaging. These methods often have inferior image spatial resolution compared to GA-enhanced HBP-T1w and may be unable to resolve small (<1cm) lesions visible only on HBP-T1w. In this work, we propose a novel phase sensitive inversion recovery (PSIR) T1w-MRI in combination with 3D radial stack-of-stars imaging and model-based water/fat separation for improved detection and characterization of small FLLs.
Introduction
Colorectal cancer (CRC) is one of the leading causes of cancer related death in the world accounting for 1.4 million new cases and over 700,000 deaths in 20121–3. According to various studies, 30 to 50% of the patients suffering from CRC, develop liver metastases, which carries a median survival of 5 to 20 months4-7.GA-enhanced, hepatobiliary phase (HBP) T1w recently became an essential tool for detection of liver metastases and focal liver lesions (FLL) in general, due to its excellent SNR performance and high resolution8,9. Successful characterization of the lesions require addition of T2w, DWI or DCE-T1w methods. However, GA-enhanced HBP-T1w often outperforms other imaging methods, where small lesions may be occult on all other sequences except GA-enhanced HBP-T1w imaging10–13. As a result, such lesions either remain undetected or characterized as “indeterminate”.
Conventional T1w imaging methods alone are insufficient for correct characterization of FLLs due to lack of a strong T1 contrast that can delineate differences between benign (eg., cyst) and malignant lesions. Magnetization preparation via inversion recovery (IR) is an effective method to increase T1 contrast, where longitudinal magnetization is inverted with a 180-degree inversion pulse prior to k-space readout (e.g., SGRE, SSFP). Phase sensitive (PS) image reconstruction methods are well-suited for IR-based imaging techniques since they can utilize the full dynamic range of the longitudinal magnetization. Importantly the performance of the PS reconstruction does not heavily rely on optimal inversion time (TI) selection14.
Cartesian k-space trajectories commonly used in clinical settings are prone to motion artifacts especially in abdominal MRI due to respiratory motion. Breath-held image acquisitions are used to minimize the motion artifacts, which limits the use of these methods in children and patients who are unable to hold their breath. Radial stack-of-stars sampling strategies, in combination with golden angle projection ordering, offer motion insensitivity and incoherent undersampling artifacts that can be leveraged in a compressed sensing reconstruction15.
In this work, we propose a novel free-breathing, chemical shift encoded (CSE), 3D PSIR HBP-T1w in combination with stack-of-stars imaging and model-based compressed sensing reconstruction for improved detection and characterization of small FLLs.
Methods
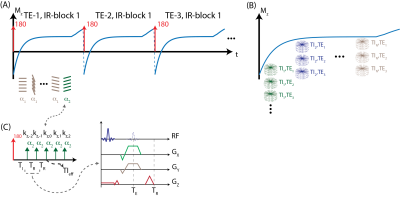
MRI Acquisition: An adiabatic inversion pulse is used to invert the longitudinal magnetization, followed by the acquisition of a series of radial stacks of single echo kz-encodes. This acquisition scheme is repeated for three-echoes for CSE-MRI. Last TI frame is acquired with a small flip angle to minimize T1 contrast on the phase reference14. Magnetization preparation and acquisition scheme is illustrated in Figure 1. Projection angles are selected according to eq.1 to i) uniformly sample each TI and TE frame and ii) create incoherent undersampling patterns in both TI and TE dimensions. $$$k, m, n $$$ denote indices of kz-stack, IR-block, and TE, respectively. $$$N_{TI}, N_{TE}$$$ and $$$PR$$$ denote number of TI, TE frames and projections.\begin{equation}
\Theta(k,m,n)~=~\begin{bmatrix}~PR~\cdot~N_{TE}~\frac{k-1}{N_{TI}}+PR~\cdot~\frac{n-1}{N_{TI}}+m-1~\end{bmatrix}~\cdot~\frac{\pi}{1.618}~\tag{1}
\end{equation}
Phase Sensitive Inversion Recovery: Three-echo, radial stack-of-stars images were acquired in one volunteer (male, age=33) on a 3.0T MR scanner (GE Healthcare Discovery MR750, Waukesha, WI) using a 32-channel phased array torso coil. Imaging parameters were as follows: FOV=380x380x130mm3, resolution=0.9x0.9x2.0mm3, flip angle=8/4o, number of slices=58, partial Fourier readout fraction=70%, TR/TE1/TE2/TE3=6.9/2.2/3/3.8ms, Bandwidth=±167kHz. 12 radial stacks were acquired after each inversion pulse. Total acquisition time was 10 minutes.
Image Reconstruction: A model-based, water/fat separated, subspace constrained, locally low rank (LLR) image reconstruction algorithm is developed that reconstructs the entire dataset jointly, taking advantage of the incoherent undersampling patterns in both TI and TE dimensions. The proposed algorithm directly provides water and fat separated subspace representations. LLR constraints are applied to water and fat subspace representations separately16. Basis functions were calculated covering a wide range of T1 values, B1 and inversion inhomogeneities. Figure 2 describes the proposed image reconstruction algorithm.
Results
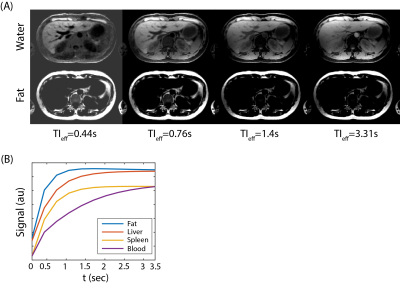
Figure 3 shows the phase sensitive, water/fat separated T1w image series at different effective TIs. Since water/fat separation is built-in to the image reconstruction, entire dataset, consisting of different TE and TI frames, is reconstructed jointly, resulting in high image quality and robust water/fat separation. Phase sensitive image contrasts are consistent with the T1 relaxations of different tissue types, i.e., at 3.0T without any contrast enhancement, subcutaneous fat shows the fastest recovery, followed by liver parenchyma, spleen, and blood.Discussion
In this work, we present a novel CSE, stack-of-stars acquisition scheme and a model-based compressed sensing reconstruction algorithm for free-breathing, 3D, PSIR imaging method. Model-based reconstruction incorporates water/fat separation into the image reconstruction, allowing for joint reconstruction of all TI and TE frames for more robust image reconstruction. Phase sensitive reconstruction captures the true phase of the spins, taking advantage of the full dynamic range of T1 contrast. The proposed stack-of-stars sampling strategy minimizes motion sensitivity against respiration, while allowing for uniform and incoherent sampling of TI and TE frames. Future work will include implementation of a respiratory gating algorithm, post-contrast enhanced imaging and studies in patients with focal liver lesions.Acknowledgements
We wish to acknowledge investigator-initiated research support from Bayer, UW Institute for Clinical and Translational Research, and the Clinical and Translational Science Award of the NCATS/NIH (UL1TR002373). Further, we wish to acknowledge GE Healthcare who provides research support to the University of Wisconsin. Finally, Dr. Reeder is a Romnes Faculty Fellow, and has received an award provided by the University of Wisconsin-Madison Office of the Vice Chancellor for Research and Graduate Education with funding from the Wisconsin Alumni Research Foundation.References
[1] M. Arnold, M. S. Sierra, M. Laversanne, I. Soerjomataram, A. Jemal, and F. Bray, “Global patterns and trends in colorectal cancer incidence and mortality,” Gut, vol. 66, no. 4, pp. 683–691, Apr. 2017, doi: 10.1136/gutjnl-2015-310912.
[2] R. L. Siegel et al., “Colorectal Cancer Incidence Patterns in the United States, 1974–2013,” JNCI: Journal of the National Cancer Institute, vol. 109, no. 8, Aug. 2017, doi: 10.1093/jnci/djw322.
[3] J. Engstrand, H. Nilsson, C. Strömberg, E. Jonas, and J. Freedman, “Colorectal cancer liver metastases – a population-based study on incidence, management and survival,” BMC Cancer, vol. 18, no. 1, p. 78, Dec. 2018, doi: 10.1186/s12885-017-3925-x.
[4] C. S. van Kessel, C. F. M. Buckens, M. A. A. J. van den Bosch, M. S. van Leeuwen, R. van Hillegersberg, and H. M. Verkooijen, “Preoperative Imaging of Colorectal Liver Metastases After Neoadjuvant Chemotherapy: A Meta-Analysis,” Ann Surg Oncol, vol. 19, no. 9, pp. 2805–2813, Sep. 2012, doi: 10.1245/s10434-012-2300-z.
[5] W. Schima, “Liver metastases of colorectal cancer: US, CT or MR?,” Cancer Imaging, vol. 5, no. special issue A, pp. S149–S156, 2005, doi: 10.1102/1470-7330.2005.0035.
[6] M. A. Choti et al., “Trends in Long-Term Survival Following Liver Resection for Hepatic Colorectal Metastases:,” Annals of Surgery, vol. 235, no. 6, pp. 759–766, Jun. 2002, doi: 10.1097/00000658-200206000-00002.
[7] E. P. Misiakos, “Current treatment for colorectal liver metastases,” WJG, vol. 17, no. 36, p. 4067, 2011, doi: 10.3748/wjg.v17.i36.4067.
[8] M. R. Bashir, D. B. Husarik, T. J. Ziemlewicz, R. T. Gupta, D. T. Boll, and E. M. Merkle, “Liver MRI in the hepatocyte phase with gadolinium-EOB-DTPA: Does increasing the flip angle improve conspicuity and detection rate of hypointense lesions?,” J. Magn. Reson. Imaging, vol. 35, no. 3, pp. 611–616, Mar. 2012, doi: 10.1002/jmri.22850.
[9] V. Vilgrain, M. Esvan, M. Ronot, A. Caumont-Prim, C. Aubé, and G. Chatellier, “A meta-analysis of diffusion-weighted and gadoxetic acid-enhanced MR imaging for the detection of liver metastases,” Eur Radiol, vol. 26, no. 12, pp. 4595–4615, Dec. 2016, doi: 10.1007/s00330-016-4250-5.[10] P. Bannas, U. Motosugi, D. Hernando, M. S. Rahimi, J. H. Holmes, and S. B. Reeder, “Combined gadoxetic acid and gadofosveset enhanced liver MRI: A feasibility and parameter optimization study: Combined Gadoxetic Acid and Gadofosveset Liver MRI,” Magn. Reson. Med., vol. 75, no. 1, pp. 318–328, Jan. 2016, doi: 10.1002/mrm.25554.
[11] U. Motosugi, P. Bannas, D. Hernando, M. Salmani Rahimi, J. H. Holmes, and S. B. Reeder, “Intraindividual Crossover Comparison of Gadoxetic Acid Dose for Liver MRI in Normal Volunteers,” MRMS, vol. 15, no. 1, pp. 60–72, 2016, doi: 10.2463/mrms.2015-0005.
[12] A. Frydrychowicz, S. K. Nagle, S. L. D’Souza, K. K. Vigen, and S. B. Reeder, “Optimized high-resolution contrast-enhanced hepatobiliary imaging at 3 tesla: A cross-over comparison of gadobenate dimeglumine and gadoxetic acid,” Journal of Magnetic Resonance Imaging, vol. 34, no. 3, pp. 585–594, Sep. 2011, doi: 10.1002/jmri.22713.
[13] S. K. Nagle et al., “High resolution navigated three-dimensional T1-weighted hepatobiliary MRI using gadoxetic acid optimized for 1.5 tesla,” J. Magn. Reson. Imaging, vol. 36, no. 4, pp. 890–899, Oct. 2012, doi: 10.1002/jmri.23713.
[14] P. Kellman, A. E. Arai, E. R. McVeigh, and A. H. Aletras, “Phase-sensitive inversion recovery for detecting myocardial infarction using gadolinium-delayed hyperenhancement,” Magn. Reson. Med., vol. 47, no. 2, pp. 372–383, Feb. 2002, doi: 10.1002/mrm.10051.
[15] L. Feng et al., “Golden-angle radial sparse parallel MRI: Combination of compressed sensing, parallel imaging, and golden-angle radial sampling for fast and flexible dynamic volumetric MRI: iGRASP: Iterative Golden-Angle RAdial Sparse Parallel MRI,” Magn. Reson. Med., vol. 72, no. 3, pp. 707–717, Sep. 2014, doi: 10.1002/mrm.24980.
[16] J. I. Tamir et al., “T2 shuffling: Sharp, multicontrast, volumetric fast spin-echo imaging,” Magnetic Resonance in Medicine, vol. 77, no. 1, pp. 180–195, Jan. 2017, doi: 10.1002/mrm.26102.
Figures