0599
Deep Learning-guided Weighted Averaging for Signal Dropout Compensation in Liver DWI1Pattern Recognition Lab, Department of Computer Science, Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, Germany, 2MR Application Predevelopment, Siemens Healthcare GmbH, Erlangen, Germany
Synopsis
Signal-dropouts caused by cardiac motion are frequently observed artifacts in diffusion-weighted imaging (DWI) of the liver. When several repetitions of a given slice are affected, uniform averaging results in locally reduced liver signal. This work proposes an adaptive weighted averaging of repetitions to locally suppress signal-dropouts. Therefore, weight maps are estimated by an algorithm which computes robust patch statistics under the guidance of a learned classifier which marks corrupted repetitions. In comparison to other methods, the proposed approach enables more homogeneous liver signal and less biased quantitative maps while sacrificing little signal-to-noise ratio (SNR).
Introduction
Despite its usefulness for detecting and assessing lesions [1,2], abdominal DWI can suffer from signal-dropouts which impede its diagnostic value. They are the result of intra-voxel dephasing primarily caused by cardiac motion during diffusion encoding with strong b-values. Since the left liver lobe is anatomically close to the heart, it is particularly prone to this type of artifact. In a standard DWI acquisition, which involves averaging of multiple repetitions for the sake of SNR, a significant portion of repetitions at high b-values can be affected by signal-dropouts. Hence, liver signal may suffer from inhomogeneity in the averaged image leading to locally biased quantitative maps as well. Motion-robust diffusion encoding can be employed to reduce the risk of signal-dropouts at the cost of a prolonged TE [3,4]. Alternatively, post-processing techniques were proposed which aim to detect and exclude affected repetitions. In [5], this was realized by training a Deep Learning classifier (DLC). While discarding corrupted repetitions can promote signal homogeneity in averaged images, SNR efficiency will inherently suffer, especially considering that signal-dropouts are typically local.The goal of this work is to estimate spatially resolved weight maps that allow local suppression of signal-dropouts while maintaining SNR in uncorrupted image regions. In contrast to our previous work [6] which used a neural network to predict weight maps from a set of repetitions directly, we aim to make this approach more interpretable and controllable by employing a conventional algorithm which assigns weights based on the deviation from a patch-based reference value. A classifier is then used to support the calculation of a more robust reference value by marking corrupted repetitions.
Methods
Adaptive Weighting Algorithm (AWA): The procedure to estimate the $$$i$$$-th pixel of the weight maps $$$\boldsymbol{w_i}=[w_{i,1},...,w_{i,N}]^T$$$ for a set of $$$N$$$ repetitions is depicted in Figure 1. By extracting patches of size $$$P\times P$$$ centered around the $$$i$$$-th pixel, $$$\boldsymbol{w_i}$$$ is determined by a scoring function $$$f(\cdot)$$$ which penalizes deviations $$$\boldsymbol{d_i}$$$ from a median-based reference value $$$m_i$$$ under a tolerance $$$s_i$$$. $$$\lambda$$$ is a manual hyperparameter which controls the smoothness of the scoring function. The normalized weights are then used to compute a weighted sum $$$\tilde{p}_i$$$ of repetitions $$$[p_{i,1},...,p_{i,N}]^T$$$ for every pixel as follows:$$\tilde{p}_i=\sum_{n=1}^N\frac{w_{i,n}}{||\boldsymbol{w_i}||}\cdot p_{i,n}\>.$$
Classification Network: Although the median-based reference value is relatively robust, it can become biased if the majority of repetitions contains signal-dropouts. Therefore, we propose to train a DLC to detect corrupted repetitions and exclude them from the calculation of $$$m_i$$$, such that a more robust median is computed on the subset of uncorrupted repetitions. The architecture of the DLC is presented in Figure 2. The Deep Set concept [7] is implemented by jointly classifying the batch of repetitions for a given slice and sharing information via batch pooling within every encoder-block. The classifier is trained by minimizing the binary cross entropy between the prediction and the ground-truth label.
Data: Free-breathing liver DWI with b-values of 50 and 800 s/mm2 (5-10 and 15-20 repetitions, respectively) was acquired in 29 volunteers on 1.5 and 3 T MR scanners (MAGNETOM, Siemens Healthcare, Erlangen, Germany) using a prototypical single-shot EPI sequence. Data was divided on a volunteer level into training, validation, and test splits (21/4/4). Depending on the occurrence of signal-dropouts, the individual repetitions of all acquired slices were manually labeled as either "clean" (negative) or "corrupted" (positive).
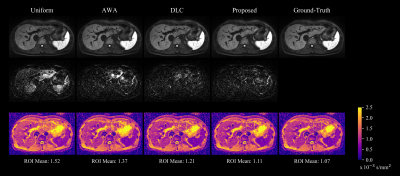
Evaluation: The proposed method was compared against three other approaches: (1) uniform averaging, (2) the AWA without the assistance of a DLC, and (3) using the DLC to simply discard corrupted repetitions from the average computation as in [5]. Ground-truth images were generated by taking the uniform average of uncorrupted repetitions only. In addition to the DW images, ADC and SNR maps were analyzed.
Results & Discussion
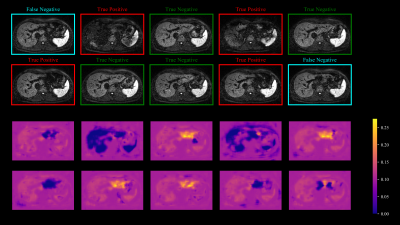
Figure 3 illustrates on a set of ten repetitions that areas of dropouts accurately coincide with lower values in the respective weight maps generated by the proposed method. The trained DLC achieved an AUC score of 0.84 on the entire test split. Although two repetitions in Figure 3 are falsely classified as negatives, the algorithm is still able to produce lower weights for the image regions affected by signal-dropouts.Applying the weight maps accordingly elevates the signal in the left liver lobe compared to uniform averaging as shown in Figure 4. The corresponding overestimation of the ADC can be alleviated by the proposed method as well. AWA without DLC-support is limited by the fact that the majority of repetitions in the set is corrupted which results in a biased median for reference. The purely DLC-based approach produces slightly attenuated signal in the left liver lobe due to false negatives contributing to the average.
Figure 5 shows the relative SNR maps of the proposed and the DLC-based approach when compared to uniform averaging. While the SNR reduces globally in the case of discarding positively classified repetitions, the proposed adaptive weighting is able to maintain SNR in most image regions and sacrifices SNR only where it is necessary to recover signal intensity.
Conclusion
We demonstrate that weight maps estimated by a DL-guided algorithm relying on patch statistics can effectively suppress local signal-dropouts in liver DWI in an SNR-efficient manner.Acknowledgements
We would like to thank 'd.hip – digital health innovation platform' for supporting this project.References
[1] Taouli, B., & Koh, D. M. (2010). Diffusion-weighted MR imaging of the liver. Radiology, 254(1), 47-66.
[2] Mitchell, D. G., Bruix, J., Sherman, M., & Sirlin, C. B. (2015). LI‐RADS (Liver Imaging Reporting and Data System): Summary, discussion, and consensus of the LI‐RADS Management Working Group and future directions. Hepatology, 61(3), 1056-1065.
[3] Ozaki, M., Inoue, Y., Miyati, T., Hata, H., Mizukami, S., Komi, S., ... & Woodhams, R. (2013). Motion artifact reduction of diffusion‐weighted MRI of the liver: use of velocity‐compensated diffusion gradients combined with tetrahedral gradients. Journal of Magnetic Resonance Imaging, 37(1), 172-178.
[4] Aliotta, E., Wu, H. H., & Ennis, D. B. (2017). Convex optimized diffusion encoding (CODE) gradient waveforms for minimum echo time and bulk motion–compensated diffusion‐weighted MRI. Magnetic resonance in medicine, 77(2), 717-729.
[5] Tamada, D., Motosugi, U., & Onishi, H (2018). Improving the image quality of liver DWI using the convolutional neural network-based selection algorithm. Proceedings of the International Society of Magnetic Resonance in Medicine, #2723.
[6] Gadjimuradov, F., Benkert, T., Nickel, M. D., & Maier, A. (2021). Deep Learning-based Adaptive Image Combination for Signal–Dropout Suppression in Liver DWI. Proceedings of the International Society of Magnetic Resonance in Medicine, #534.
[7] Zaheer, M., Kottur, S., Ravanbakhsh, S., Poczos, B., Salakhutdinov, R. R., & Smola, A. J. (2017). Deep sets. Advances in neural information processing systems, 30, 3391-3401.
Figures