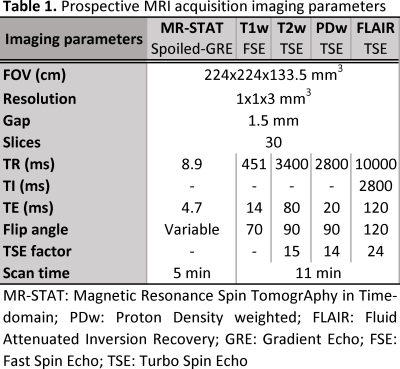
0597
Synthetic MRI with MR-STAT: results from a clinical trial1Computational Imaging Group for MR diagnostic and therapy, Center for Image Sciences, University Medical Center Utrecht, Utrecht, Netherlands, 2Department of Radiotherapy, University Medical Centre Utrecht, Utrecht, Netherlands, 3Department of Neurosciences, University of Turin, Turin, Italy, 4Department of Radiology and Nuclear Medicine, University Medical Centre Utrecht, Utrecht, Netherlands
Synopsis
Magnetic Resonance Spin TomogrAphy in Time-domain (MR-STAT) reconstructs multiple quantitative MR parameters from a single fast scan. This quantitative information can be leveraged for several purposes, including the synthetization of clinically desired image contrasts. Preliminary results of the first clinical trial using MR-STAT in patients with neurological diseases show that the synthetically generated contrast images (i.e., T1w, T2w, PDw and FLAIR) were acceptable for diagnostic use (5-point Likert scale ≥ 3), although the score was lower compared to conventional contrasts. This highlights the potential of MR-STAT to provide synthetic contrasts on top of quantitative maps, while reducing scan time.
Introduction
A standard clinical MRI neuro-examination consists of several sequences with different image contrast weightings (e.g., T1w, T2w, proton density [PD], fluid-attenuated inversion recovery [FLAIR]), totaling an acquisition time between 15 – 20 minutes. Using the Magnetic Resonance Spin TomogrAphy in Time-domain (MR-STAT) technique, the quantitative value of T1, T2 and proton density can be extracted with one 5-minute multi-parametric whole-brain sequence (1, 2). MR-STAT consists of a transient-state Cartesian acquisition scheme and model-based non-linear inversion reconstruction. The reconstructed quantitative values can be used in diagnosis, evaluation of disease progression and treatment (3, 4). Additionally, any desired image contrast can be synthesized according to a physical signal model. The aim of this prospective cross-sectional study was to assess the diagnostic image quality of MR-STAT-generated synthetic image data sets in healthy participants and patients with neurological diseases compared to conventional images (5).Methods
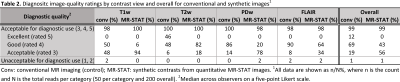
In total 50 participants (22 females) with a median age of 45 years (range: 21 – 79 years) were included: 10 healthy volunteers without a history of neurological disease and 40 patients with one of four common neurological diseases – brain tumor, epilepsy, multiple sclerosis (MS) or ischemic stroke – equally divided among these disease groups. MRI exams were performed at the University Medical Center Utrecht on a 3T MR system (Ingenia, Philips, Best, the Netherlands); all subjects underwent one MR-STAT sequence and four clinically used T1w, T2w, PD and FLAIR MRI sequences. The 5-minute-long MR-STAT sequence was a Cartesian encoded, spoiled Gradient Echo with a slowly varying flip angle preceded by a non-selective inversion pulse (2). The specific sequence parameters for MR-STAT, which was 54% faster, and conventional sequences are specified in Table 1. The reconstructed quantitative MR images were used to generate corresponding synthetic contrast images from standard signal models after denoising the parameter maps using deep learning (6). For these preliminary results, a total of 400 (4 synthetic MR-STAT and 4 conventional for each of the 50 participants) randomized images were assessed by at least two out of three blinded independent neuroradiologist (>6 years’ experience). Assessment sessions included 100 unique images and were separated by a memory-washout period of at least two weeks. Images were scored for overall quality on a 5-point Likert scale, morphologic legibility and presence/absence of artifacts.Results and Discussion
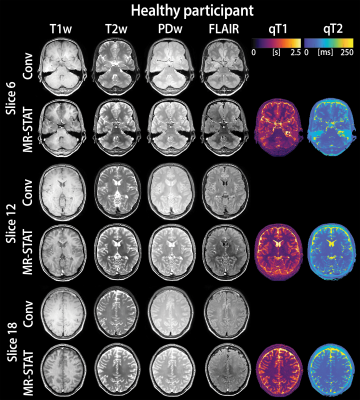
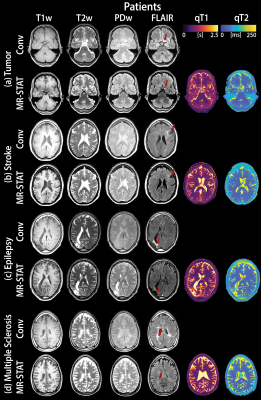
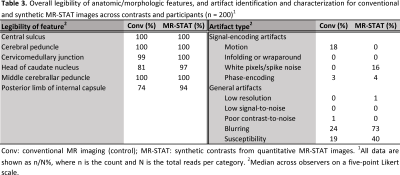
The overall image quality was acceptable for diagnostic use (Likert scale ≥ 3) for 99% of all contrasts synthesized from quantitative MR-STAT as well as for conventional serial acquisition (Table 2). However, synthetic MR-STAT images scored lower than conventional for T1w, PDw and FLAIR contrasts (the exception being T2w) (Table 2). Images synthetically generated from quantitative MR-STAT maps and conventional acquisitions of healthy participants had comparable image contrasts, an example is shown in Figure 1. Additionally, similar image quality can be observed in synthetic MR-STAT and conventional images in the relevant slice of a patient with a (a) brain tumor, (b) stroke, (c) epilepsy and (d) MS (Figure 2). However, in some patients (see Figure 2) we noticed that lesions appear hypointense in synthetic MR-STAT FLAIR images, while they are hyperintense in conventional images. Inferior image quality of synthetic image contrasts were also previously observed (7-9), but could be enhanced as shown by Hagiwara et al. (10). Legibility of prior determined anatomic/morphologic features were similar for the synthetic and conventional images, except for the head of the caudate nucleus and the posterior limb of the internal capsule which were more legible in the synthetic MR-STAT T1w images (Table 3). The overall percentage of images with artifacts identified and characterized for conventional and synthetic MR-STAT images is shown in Table 3. In the conventional images, more motion artifacts were observed (Δ 18%) predominantly in the FLAIR contrast (Δ 50%). In contrast, more images with synthetic MR-STAT T1w contrast had white pixels/spike noise (Δ 62%), while overall more blurring (Δ 49%), susceptibility (Δ 21%) and flow effects were observed. Flow effects are intrinsic to the 2D gradient echo MR-STAT acquisition technique, while optimization of the synthetization process may reduce the blurring effects. A possible alternative to improve synthetized contrasts could be machine-learning based methods (11).Conclusion
The synthetic MR-STAT images from a single fast scan were overall acceptable for diagnostic use, although the perceived image quality is lower and in some cases the contrast of lesions differ in FLAIR images. These results highlight the potential of MR-STAT to provide clinicians with clinically useful image contrasts within substantially reduced scan time, in addition to multi-parametric quantitative maps.Acknowledgements
We would like to thank neuroradiologists Dr. ir. E.P.A. Vonken, Dr. J.W. Dankbaar from the University Medical Center Utrecht and Dr. V.C.W. Keil from the Amsterdam University Medical Center for assessing the MR images. Additionally, we thank Carlo Lucci from the University Medical Center Utrecht for supporting the data acquisition.
This clinical trial was funded by the Netherlands Organization for Scientific Research (NWO; Demonstrator Grant 16937), and has been registered in the Netherlands Trial Register with number NL8437 (https://www.trialregister.nl/trial/8437)
References
1. Sbrizzi A, Heide OVD, Cloos M, Toorn AVD, Hoogduin H, Luijten PR, et al. Fast quantitative MRI as a nonlinear tomography problem. Magn Reson Imaging. 2018; 46: 56-63.
Figures