0590
Non-invasive metabolic biomarkers of interneuron cell transplantation therapy in an epilepsy model1Department of Physical Therapy and Rehabilitation Science, University of California San Francisco, San Francisco, CA, United States, 2Department of Radiology and Biomedical Imaging, University of California San Francisco, San Francisco, CA, United States, 3Neurona Therapeutics, San Francisco, CA, United States
Synopsis
Interneuron transplantation targeting GABAergic network dysfunction is a novel alternative therapeutic approach for chronic focal epilepsy. However, in vivo monitoring of response to such cellular therapeutics remains challenging. Here, we combined high resolution in vivo and ex vivo 1H MRS to investigate the metabolic alterations in epileptic hippocampus in a preclinical model of chronic mesial temporal lobe epilepsy, and the effect of human inhibitory interneuron transplantation on metabolism. We discovered that hippocampal levels of GABA, NAA/Cr, GLX/Cr, GABA/GLX and GABA/NAA were reversed to healthy control levels in epileptic mice transplanted with inhibitory interneurons, providing unique biomarkers of therapeutic response.
Introduction
According to the World Health Organization, epilepsy is the most common chronic neurological disease affecting approximately 1% of the human population of all ages worldwide1,2. One-third of epileptic people live with recurrent uncontrollable seizures because of resistance to existing anti-seizure drugs. Dysfunction of GABAergic inhibitory interneurons due to hyperexcitability of neuronal networks has been linked to various neurological disorders including epilepsy3. Previous preclinical studies in animal models of temporal lobe epilepsy (TLE) have shown that intrahippocampal transplantation of GABAergic interneurons can suppress seizures and ameliorate behavioral deficits, providing a potential alternative therapeutic strategy for people with drug-resistant focal epilepsy4,5. In this study, we first characterized the metabolic profile of the mouse epileptic hippocampus using high resolution in vivo 1H MRS at 14.1T and ex vivo 1H MRS at 800MHz on paired samples. We further applied this approach to epileptic animals transplanted with either human interneurons or vehicle, and we identified a set of metabolites and metabolic ratios that are altered in response to inhibitory interneuron cellular therapy.Methods
TLE mouse model: Unilateral injection of kainic acid into the right dorsal hippocampus of immunocompromised male mice (NOG; NOD.Cg-PrkdcscidIl2rgtm1Sug/JicTac; 5-6 weeks old) induced status epilepticus, that subsequently led to epilepsy as confirmed by observation of spontaneous recurrent seizures. Approximately 4-6 weeks after kainate induction, epileptic animals received either human pallial-type interneurons derived from pluripotent stem cells, or vehicle control, via intrahippocampal injection. Studies were performed on two time points: 1) 30 days post-epilepsy induction (epileptic (n=10) and age-matched healthy control (n=10) mice); 2) 8 months post-epilepsy induction (epileptic injected with either vehicle (n=5) or interneurons (n=9), and age-matched healthy controls (n=6)). Figure 1 illustrates our study design. Researchers were blinded to treatment cohorts.In vivo 1H MRS: In vivo studies were performed in vertical Agilent 14.1T scanner equipped with a 1H volume coil (40mm diameter). T2-weighted images were acquired using a fast spin-echo multi-slice sequence (ESP=9.81ms, TR=3000ms, 32/8, FOV=25x25mm2, 256x256, slice thickness=0.4mm, gap=0.1mm, NA=4-6). In vivo 1H MRS spectra were recorded from a single 1.2x2.5x2.0mm3 voxel placed on the right dorsal hippocampus using the PRESS pulse sequence (TE/TR=20/2000ms, 512 averages, VAPOR water suppression) and analyzed using LCmodel with scaling to water signal. The following metabolites were identified and quantified: N-acetyl-aspartate (NAA) and N-acetyl-aspartyl-glutamate (NAAG), tNAA (NAA+NAAG), choline (Cho, sum of choline-containing metabolites), creatine (Cr, sum of creatine and phosphocreatine), myo-inositol (mI), taurine (Tau), lactate (Lac), 4-aminobutyric acid (GABA), glutamate (Glu), glutamine (Gln), GLX (Glu+Gln).
Ex vivo 1H MRS: After in vivo MRS the animals were euthanized and perfused with ice-cold PBS. Brain tissues were collected, and dissected hippocampi were snap frozen in liquid nitrogen for further MeOH/Chloroform extraction. Lyophilized samples were dissolved in D2O and 1H MRS spectra were acquired using 1D-gradient NOESY sequence at Bruker 800MHz magnet following analysis using Chenomx with normalization to wet weight.
Statistics: Results represent mean ± stdev. Two-way-Anova was used to determine statistical significance of differences, and p-value ≤ 0.05 was considered significant.
Results and Discussion
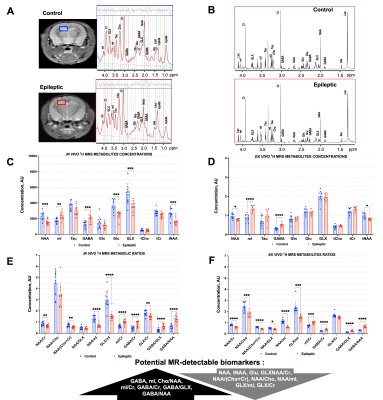
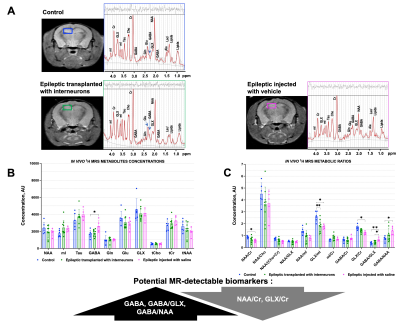
The TLE mouse model exhibits spontaneous recurrent mesiotemporal seizures and hippocampal tissue damage, which resemble human mesial TLE focal seizures and sclerosis. At 30 days post kainate induction, in vivo 1H MRS acquired from a single voxel revealed that induction of epilepsy led to a significant decrease in NAA, tNAA, NAA/Cr, NAA/(Cho+Cr), NAA/myoinositol, and GLX/myoinositol, and a significant increase in GABA, myoinositol, myoinositol/Cr, GABA/Cr, GABA/GLX and GABA/NAA in epileptic hippocampus when compared to corresponding controls (Figure 2A,C,E). Importantly, these findings were further confirmed by paired ex vivo analysis of metabolites extracted from the hippocampi of these animals (Fig 2B,D,F).Next, we investigated the functional response to interneuron transplantation in the TLE mouse model at 8 months post-epilepsy induction. At that time, more than two-thirds of cell-treated animals become seizure-free and display significantly reduced dentate granule cell layer dispersion and hippocampal pathology. We assessed whether MR-detectable metabolites, found to be altered in the epileptic mouse hippocampus, were corrected in response to interneuron transplantation. We first quantified in vivo 1H MRS hippocampal metabolic profiles of epileptic mice injected with vehicle and demonstrated a significant decrease in NAA/Cr, GLX/myoinositol, GLX/Cr and significant increase in GABA, GABA/GLX and GABA/NAA when compared to healthy controls (Figure 3). Moreover, these metabolic alterations were sustained for the duration of the study, 8 months post-epilepsy induction. In contrast, post interneuron transplantation, the majority of the metabolic changes that we found altered in epileptic mice treated with vehicle, were corrected towards the levels found in age-matched healthy control mice (Figure 3). Importantly, the metabolic changes following human interneuron transplantation were associated with focal seizure suppression and reduced hippocampal pathology.
Conclusion
To conclude, our study identified GABA, NAA/Cr, GLX/Cr, GABA/GLX and GABA/NAA as potential translatable metabolic biomarkers of inhibitory interneuron cell therapy. These metabolites and metabolic ratios were significantly altered in chronic epileptic mice injected with vehicle but were reversed to the levels of healthy hippocampus in epileptic mice transplanted with GABAergic interneurons. Here, we present non-invasive MR-detectable biomarkers to consider in potential future clinical studies of interneuron cell therapy as well as other anti-seizure drugs.Acknowledgements
This research was funded by an industry-academia partnership between the Chaumeil lab at UCSF and Neurona Therapeutics.References
1. Duncan, J. S., Sander, J. W., Sisodiya, S. M. & Walker, M. C. Adult epilepsy. Lancet, (2006); 367: 1087–100.
2. World Health Organization (WHO). Epilepsy: A public health imperative (2019) - SUMMARY. International League Against Epilepsy (ILAE).
3. Rossignol, E. Genetics and Function of Neocortical GABAergic Interneurons in Neurodevelopmental Disorders. Neural Plasticity, (2011), doi.org/10.1155/2011/649325.
4. Southwell, D. G., Nicholas, C.R., Basbaum, A.I., Stryker, M.P., Kriegstein, A.R., Rubenstein, J.L., Alvarez-Buylla A.. Interneurons from Embryonic Development to Cell-Based Therapy. Science, (2014), 344(6180), doi:10.1126/science.1240622.
5. Hunt, R. F., Girskis, K. M., Rubenstein, J. L., Alvarez-Buylla, A. & Baraban, S. C. GABA progenitors grafted into the adult epileptic brain control seizures and abnormal behavior. Nature Neuroscience, (2013), 16: 692–697, doi:10.1038/nn.3392.
Figures