0589
Multimodal Lesion Phenotyping Improves Seizure Outcome Classification after Traumatic Brain Injury: An EpiBioS4Rx Study
Rachael Garner1, Alexis Bennett1, Akul Sharma1, Michael Douglas Morris2, Marianna La Rocca1,3, Giuseppe Barisano1, Ruskin Cua4, Paul Vespa2, Arthur W Toga1, and Dominique Duncan1
1USC Stevens Neuroimaging and Informatics Institute, Keck School of Medicine, University of Southern California, Los Angeles, CA, United States, 2David Geffen School of Medicine, University of California, Los Angeles, Los Angeles, CA, United States, 3Dipartimento Interateneo di Fisica, Università di Bari, Bari, Italy, 4USC Department of Radiology, Keck School of Medicine of USC, University of Southern California, Los Angeles, CA, United States
1USC Stevens Neuroimaging and Informatics Institute, Keck School of Medicine, University of Southern California, Los Angeles, CA, United States, 2David Geffen School of Medicine, University of California, Los Angeles, Los Angeles, CA, United States, 3Dipartimento Interateneo di Fisica, Università di Bari, Bari, Italy, 4USC Department of Radiology, Keck School of Medicine of USC, University of Southern California, Los Angeles, CA, United States
Synopsis
Posttraumatic epilepsy (PTE), or recurrent seizures after traumatic brain injury (TBI), is a debilitating complication of TBI. We present a multimodal approach to classify seizure outcomes using computed tomography (CT) and magnetic resonance imaging (MRI) features that characterize lesion phenotypes. Five logistic regression models to predict seizure outcome are presented, using patient demographics and clinical information in conjunction with CT and MRI variables that describe lesion characteristics including contusion type and location as well as whole-brain lesion volumetrics. The optimal model utilized all four categories of features, yielding 91.4% sensitivity, 75% specificity, and 0.886 area under the curve performance.
Introduction
Traumatic brain injuries (TBIs) affect 2.5 million Americans annually, with over 300,000 severe injuries resulting in emergency room visits and hospital admissions.1 Posttraumatic epilepsy (PTE), or recurrent seizures more than one week after TBI, is a debilitating complication of TBI. Estimated incidence ranges from 2%-50% largely based on severity of injury, among other factors.2 The latency period prior to seizure onset allows researchers to investigate biomarkers of epileptogenesis that could predict which patients are most susceptible to developing PTE.Prior work has investigated whether phenotypic characteristics of TBI lesions on magnetic resonance imaging (MRI) and computed tomography (CT) are related to PTE outcome following TBI. For example, contusions and temporal lobe localization have been associated with increased risk of PTE.3 One recent study of 57 TBI patients also showed how using imaging features in combination with demographic and clinical variables can increase seizure classification performance.4 The goal of this work is to establish whether multimodal lesion phenotyping can help classify seizure outcomes in the Epilepsy BioInformatics Study for Antiepileptogenic Therapy (EpiBioS4Rx).
Methods
EpiBioS4Rx enrollment criteria include frontal and/or temporal hemorrhagic contusion and Glasgow Coma Scale (GCS) score between 3-13 without continuous sedation at the time of enrollment. CT and T2-Weighted-Fluid-Attenuated Inversion Recovery (T2-FLAIR) MRI were acquired for 146 EpiBioS4Rx subjects. Manually traced lesion segmentations on T2-FLAIR were produced using ITK-SNAP with two class labels for lesion core and edema (Fig.1).5 Lesion segmentations were validated by a board-certified physician with expertise in neuroradiology.Seizure outcomes were available for 59 patients, with binary labels for seizure-positive (at least one seizure after 7 days post-injury) and seizure-negative (no seizures within the 2-year follow-up window). The seizure-negative group was composed of 35 subjects (29 male, 6 female) with average age=40.46 (SD=21.4), average GCS=8.97 (SD=4.57), and average MRI acquisition at 10.0 (SD=7.48) days post-injury. The seizure-positive group was composed of 24 subjects (19 male, 5 male) with average age=44.21, average GCS=7.54 (SD=3.84), and average MRI acquisition at 13.54 (SD=7.17) days post-injury. For both groups, CT was acquired on the day of hospital admission.
In order to explore the relative importance of different variable categories, 29 variables were placed into 4 groups: a) demographics (age, sex, days post-injury of the MRI acquisition; b) clinical data (GCS at hospital admission); c) MRI-based characteristics extracted from lesion segmentations (voxel- and volume-wise size of lesion core and edema, total lesion size, lesion-to-edema ratio, number of independent lesions); and d) CT-based characteristics describing the types (hemorrhagic, subcortical, non-hemorrhagic, probable brain laceration, deep brain structure, cortical), hemispheres (left or right), and locations of contusions (Frontal, Parietal, Temporal, or Occipital lobe; Internal Capsule; Thalamus/Basal Ganglia, Midbrain, Pons, Medulla, Cerebellum).
Five binary logistic regression models were designed to distinguish seizure outcome: Model 1 used demographic information only; Model 2 used demographic and clinical data; Model 3 used demographic, clinical, and MRI-based lesion data; Model 4 used demographic, clinical, and CT-based contusion characteristics; Model 5 used demographics, clinical data, MRI-based lesion data, and CT-based contusion characteristics.
Results
Model sensitivity, specificity, and area under the curve (AUC) were as follows: Model 1 - sensitivity=85.7%, specificity=33.3%, AUC=0.656; Model 2 - sensitivity=82.8%, specificity=41.7%, AUC=0.701, Model 3 - sensitivity=85.7%, specificity=50%, AUC=0.787, Model 4 - sensitivity=82.9%, specificity=62.5%, AUC=0.808, Model 5 - sensitivity=91.4%, specificity=75%, AUC=0.886 (summary in Fig.2). A confusion matrix for Model 5 is shown in Fig.3, and AUC performance for all models is shown in Fig.4.Discussion
The highest model sensitivity, specificity, and AUC for seizure classification was found from Model 5, which combined demographic, clinical, MRI, and CT variables. Although sensitivity remained relatively consistent across models, the specificity and AUC notably increased with the addition of imaging features. Increasing specificity is especially critical when considering these results in the context of identifying patients as candidates for antiepileptogenic therapies: a high false positive rate would propose patients with low PTE risk as good candidates for potentially unnecessary interventions.The high performance of Model 5 also suggests that a multimodal approach to identifying imaging biomarkers of epileptogenesis may yield synergistic results. Frontal and temporal contusions are a widely recognized risk factor for PTE, but the addition of variables describing the full scope of lesion burden seems to add additional context that improves the specificity of seizure outcome classification. This improvement may also be attributed to the inclusion of imaging features acquired at both acute (day of hospital admission) and more chronic (~10-14 days post-injury) time points.
Conclusions
Introducing both CT- and MRI-based characteristics describing TBI phenotypes improved seizure outcome classification. Multimodal phenotyping that includes whole-brain lesion characteristics and nuanced radiological annotations regarding contusions will ideally produce highly sensitive and specific seizure prediction.Acknowledgements
Our work is supported by NINDS U54 NS100064, and is disseminated on behalf of The Epilepsy Bioinformatics Study for Antiepileptogenic Therapy (EpiBioS4Rx) investigators.References
- Centers for Disease Control and Prevention (CDC). Report to Congress on Mild Traumatic Brain Injury in the United States: Steps to prevent a serious public health problem. Centers for Disease Control and Prevention; 2003.
- Garner R, La Rocca M, Vespa P, Jones N, Monti M, Toga AW, Duncan D. Imaging biomarkers of post traumatic epileptogenesis. Epilepsia 2019;00:1–12.
- Tubi MA, Lutkenhoff E, Blanco MB, et al. Early seizures and temporal lobe trauma predict post-traumatic epilepsy: A longitudinal study. Neurobiol Dis 2018;123:115–21.
- Lutkenhoff E, Shrestha V, Ruiz Tejeda J, Real C, McArthur D, Duncan D, La Rocca M, Garner R, Toga AW, Vespa P. Early brain biomarkers of post-traumatic epilepsy: Initial report of the multicenter Epilepsy Bioinformatics Study for Anti-epileptogenic Therapy (EpiBioS4Rx) prospective study. Journal of Neurology, Neurosurgery, and Psychiatry. 2020 Nov;91(11):1154-1157.
- Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage 2006 Jul 1;31(3):1116-28.
Figures
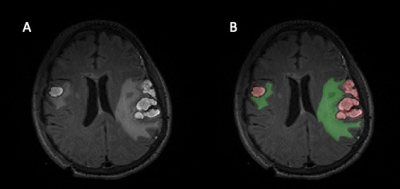
Figure 1: A) T2-Weighted-Fluid-Attenuated Inversion Recovery (T2-FLAIR) for one patient B) The accompanying lesion segmentation performed in ITK-SNAP for this subject. The hemorrhagic lesion core is labeled in red and the surrounding edema is labeled in green.
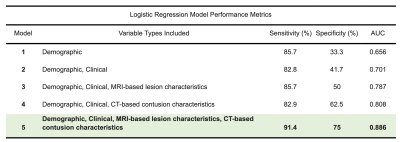
Figure 2: A summary of sensitivity, specificity, and area under the curve (AUC) values for all five tested models.
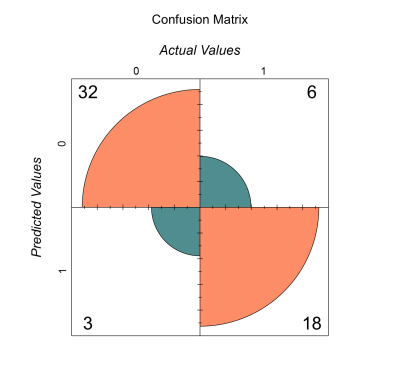
Figure 3: Confusion Matrix for Model 5, which yielded the highest sensitivity (91.4%) and specificity (75%) of all tested models. Model 5 included variables describing demographics, clinical data, MRI-based lesion data, and CT-based contusion characteristics.
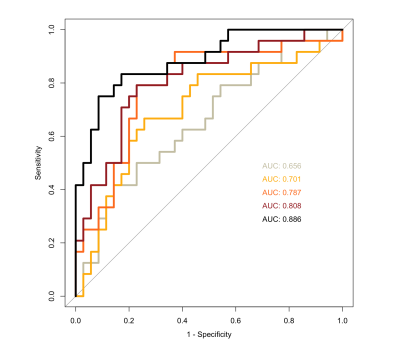
Figure 4: Area under the curve performance comparison for the five models tested.
DOI: https://doi.org/10.58530/2022/0589