0573
Family of Novel Hyperpolarized 13C Molecular Imaging Probes Monitors Real-Time Oxidative Stress in Cancer Metabolism
Kazutoshi Yamamoto1, Yohei Kondo2, Ana Opina3, Deepak Sail3, Natarajan Raju3, Burchelle Blackman3, Ronja M. Malinowski4, Tomohiro Seki1, Shun Kishimoto1, Nobu Oshima1, Yasunori Otowa1, Kota Yamashita1, Daniel R. Crooks1, Jeffrey R. Brender1, Hiroshi Nonaka2, Yutaro Saito2, Peter L. Choyke1, Jan H. Ardenkjær-Larsen 4, James B. Mitchell1, W. Marston Linehan1, Rolf Swenson3, Shinsuke Sando2, and Murali C. Krishna1
1National Cancer Institute, National Institutes of Health, Bethesda, MD, United States, 2Department of Chemistry and Biotechnology, Graduate School of Engineering, The University of Tokyo, Bunkyo-ku, Japan, 3National Heart, Lung, and Blood Institute, National Institutes of Health, Rockville, MD, United States, 4Department of Health Technology, Technical University of Denmark, Lyngby, Denmark
1National Cancer Institute, National Institutes of Health, Bethesda, MD, United States, 2Department of Chemistry and Biotechnology, Graduate School of Engineering, The University of Tokyo, Bunkyo-ku, Japan, 3National Heart, Lung, and Blood Institute, National Institutes of Health, Rockville, MD, United States, 4Department of Health Technology, Technical University of Denmark, Lyngby, Denmark
Synopsis
Molecular imaging can personalize cancer therapies by monitoring treatment responses, where hyperpolarized MRI is becoming an increasingly critical methodology. However, the limited number of available probes, which can interrogate key physiology/biochemistry related to targeted diseases, remains one of the major challenges in this growing field. In this presentation, we successfully demonstrate, for the first time, the advantages of newly synthesized 13C-Glutathione and 13C-N-acetyl cysteine probe in vivo redox status in a complementary manner. This approach serves as powerful non-invasive biomarkers to assess oxidative stress, which plays a vital role in various diseases and can be translatable to the clinical diagnosis.
Purpose
Balance between oxidant production and antioxidant defenses in cells is crucial to maintain optimal cellular functions and its redox environment. Thus, the loss of the balance, namely oxidative stress, can be commonly observed in a wide variety of human diseases. In particular, oxidant production, predominantly reactive oxygen species(ROS), is closely linked to the genesis and progression of numerous pathological conditions, including cancer, aging, diabetes, obesity, neurodegeneration, age-related retinopathy, cochlear degeneration, and chronic inflammatory diseases.1 To detect these illnesses and assess their early readout of therapeutic responses, monitoring in situ oxidative stress and in vivo redox environment can be a powerful diagnostic strategy. Imaging redox environment is particularly important for tumor tissues, since this milieu can be a critical factor for treatment responses towards chemotherapeutic pharmaceuticals, radiation therapies, and bioreductive hypoxic cell cytotoxins.2 However, such a non-invasive molecular imaging of oxidative stress is hampered by difficulties in visualizing and quantifying real-time ROS metabolisms with high selectivity, sensitivity, spatial-, temporal- resolutions. While these are challenging tasks for most techniques, clinically used hyperpolarized(HP) MRI and MRS offer a promising approach for non-invasive molecular imaging as they have advantages in their high selectivity and spatial resolutions. Previous studies successfully utilized dehydroascorbic acid, benzoylformic acid, and thiourea to monitor redox status,3-6 however, these probes may exhibit issues in either in vivo biocompatibility or translation to clinics due to their adverse effects.7 In this presentation, we overcome these disadvantages by developing novel probes to monitor in vivo oxidative stress, including glutathione, the ubiquitous most abundant thiol in animal cells(Figure 1), and N-acetyl cysteine(NAC), an FDA-approved free radical scavenger as promising candidates for in vivo HP13C-MRI. Here, we successfully synthesized and demonstrated both 13C GSH and [1-13C]NAC for in vivo HP13C-MRI with optimal level of polarization using healthy mice and human tumor xenografts. The biodistribution of these HP-probes demonstrates that a significant level of in vivo signals and their transformation can be observed globally in vivo. Therefore, hyperpolarized GSH and NAC can provide imaging assessment of monitoring site-specific redox status, antioxidant and enzymatic activities in complementary manners. Established labeling strategies of probes, which have the relatively larger molecular weight, and experimental results can lead to promote that the strategic designing of labeling schemes in endogenous biomolecules and biocompatible pharmaceuticals for HP-MRI. This enables the potential clinical diagnostic applications and in vivo studies of biochemistry to monitor their clinically relevant metabolic activities using.Methods
Hyperpolarization: 35 μl of 1.5 M13C GSH with 15 mM OX063 or 3.2 M [1-13C]NAC with 17 mM OX063 were hyperpolarized using either the SPINlab(GE Healthcare) or a Hypersense DNP polarizer(Oxford Instruments). Each hyperpolarized sample was rapidly dissolved in 4 ml of a superheated DPBS dissolution buffer, containing 100 mg/L ethylenediaminetetraacetic acid.Results and Discussion
Both 13C GSH and [1-13C]NAC were rationally designed to have optimum performances in in vivo HP-13C MRI, including elongating the T1 relaxation times particularly because GSH has one of the largest molecular weight as a dissolution DNP probe for in vivo use. The T1 relaxation times of each probes were improved to 50 seconds with deuterated 13C GSH, compared to 39 seconds with nondeuterated [1-13C]NAC including corrections for flip-angle loss. Considering the differences in the molecular weight between GSH (M.W.424) and NAC (M.W.164), the significant enhancement in the T1 relaxation time was achieved based on the strategic design of the probe(Figure1(B)).Despite its biological importance and medical relevance, the only available GSH molecular probes were demonstrated using fluorescence with limited success in only in vitro and in cell conditions.8 We also have been developing a g-glutamyl transpeptidase(GGT) probe, a GSH analog probe, however, it had disadvantages for in vivo applications on tumor xenografts(Figure2(A)). Here, we have newly developed a 13C-labeled GSH probe for HP-13C MRI experiments(Figure2(B)). To our knowledge, this is the first study directly detecting real-time enzymatic activities of both GGT and dipeptidases, which are essential metabolic pathways for the intracellular intake of GSH, indicating in vivo redox status of cells/tissues(Figure 2(C)(D)).
HP[1-13C]NAC was also used for effectively probing redox status in both in vitro and in vivo. It sensitively monitored the differences in both the NAC-GSH productions and the NAC-GSH/NAC ratios between the human pancreatic tumor cell lines as consistent with our previous report(Figure(A)-(G)).8 Chemical Shift Imaging results monitor site-specific chemical conversions of HP[1-13C]NAC in human pancreatic tumor of Hs766t(Figure 4(A)) and SU.86.86.(Figure 4(B)), as well as the orthotopic tumor of GBMJ1 tumor model(Figure4(C)).
Conclusions
To our knowledge this is the first study monitoring each step of in vivo GSH intracellular intake, which gives us insight into the redox status in the tissues/cells at the molecular level. In addition, this study effectively exhibits the general guideline for the rational designs of HP-MRI probes utilizing endogenous biomolecules and biocompatible pharmaceuticals, which have larger molecular weight. Successful comparison of newly designed probes allows us to monitor tumor metabolic activities related to oxidative stress in a complemental manner. Therefore, non-invasive molecular imaging with various metabolically inter-related probes can potentially contribute to better prognostics, detailed tumor characterizations, and timely response monitoring in cancer treatments.Acknowledgements
This study was supported by intramural research program at NCI/NIH.References
[1] Meister, A. et al., Glutathione, Ann. Rev. Biochem. 1983;52:711-760.[2] Kuppusamy, P. et al., Noninvasive imaging of tumor redox status and its modification by tissue glutathione levels., Cancer Res. 2002; 62(1):307-312.
[3] Keshari, K. R. & Wilson, D. M., Chemistry and biochemistry of 13C hyperpolarized magnetic resonance using dynamic nuclear polarization., Chem. Soc. Rev. 2014; 43:1627-1659.
[4] Keshari, K. R. et al., Hyperpolarized 13C dehydroascorbate as an endogenous redox sensor for in vivo metabolic imaging., Proc. Natl. Acad. Sci. U.S.A. 2011; 108(46):18606-18611.
[5] Wibowo, A. et al., Real-time in vivo detection of H2O2 using hyperpolarized 13C-thiourea., ACS Chem. Biol. 2017; 12:1737-1742.
[6] Lippert, A. R. et al., A hydrogen peroxide-responsive hyperpolarized 13C MRI contrast agent., 2011; 133:3776-3779.
[7] Patterson, J. W. & Lazarow, A., Sulfhydryl protection against dehydroascorbic acid diabetes., J. Biol. Chem. 1950; 186(1):141-144.
[8] Chen, X., et al., Fluorescent and colorimetric probes for detection of thiols., Chem. Soc. Rev. 2010; 39(6):2120-2135.
[9] Yamamoto, K. et al. Molecular Imaging of the Tumor Microenvironment Reveals the Relationship between Tumor Oxygenation, Glucose Uptake, and Glycolysis in Pancreatic Ductal Adenocarcinoma., Cancer Res. 2020; 80:2087-2093.
Figures
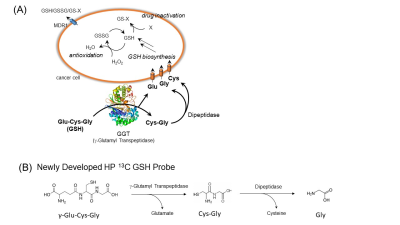
A critical role of GSH metabolism and its cellular defensive mechanism (A). A rational design of 13C GSH probe enables us to observe enzymatic pathways of GSH on the extracellular membrane protein, g-Glutamyl Transpeptidase, and dipeptidases (B). GSSG: glutathione disulfide, GS-X: glutathione-S-conjugates, MDR1: multiple drug resistance 1.
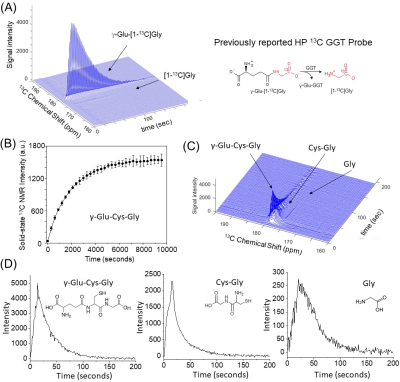
Real-time monitoring of in vivo GSH metabolism using a HP-GSH analog (g-Glu-[1-13C]Gly) and a HP-13C GSH probe (g-Glu-Cys-Gly) on a 3T MRI. (A) 13C MRS of HP-GSH analog, which only detects the one step of GGT enzymatic activities, and produces smaller levels of the metabolite on a MiaPaCa-2 tumor xenograft. (B) Optimized sample for HP-MRI shows excellent HP build-up curves of newly developed 13C GSH probe (n=3). (C) MRS indicates two metabolites from each enzymatic step on a healthy mouse body region, including kidneys and liver. The time-dependent dynamics of metabolites (D).
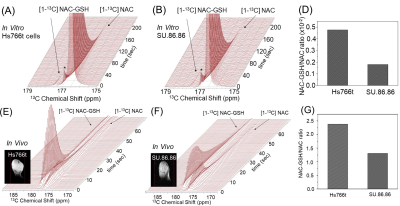
Effectively probing redox status in tumor cells and xenografts utilizing HP13C NAC. in cell NMR of HP [1-13C] NAC at 1T NMR on pancreatic tumor cells of Hs766t (A) and SU.86.86. (B) shows differences in the NAC-GSH/NAC ratios (D). These results are consistent with in vivo HP 13C NAC MRS at 3T MRI on xenografts of Hs766t (E) and SU.86.86. (F). Differences in readouts reflect on the redox status in each tumor (G). A 13C NMR peak with asterisk (*) is the natural presence of dimeric from of NAC. Tumor xenografts were formed by injecting 3×105 cells, subcutaneously into the right hind legs of mice.
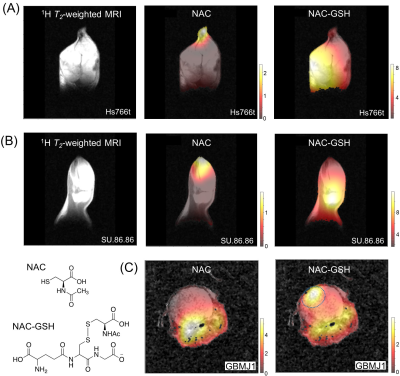
Metabolic imaging exhibits the global distribution throughout the tumor, and site-specific differences in chemical conversions of HP [1-13C] NAC. 13C chemical shift images (CSIs) of HP 13C NAC at a Philips Achieva 3T MRI on tumor xenografts of Hs766t(A), SU.86.86(B), and GBMJ1(C). Distribution maps of chemical conversion of products ((A), (B), and (C) right). In the orthotopic xenograft mouse model of the glioblastoma multiforme tumor (GBMJ1) is located in the light blue area of NAC-GSH image ((C), right). CSIs were acquired 30 seconds after the beginning of the HP NAC injections.
DOI: https://doi.org/10.58530/2022/0573