0572
Evaluating hyperpolarized [1-13C]pyruvate uptake for predicting response to stereotactic radiosurgery in brain metastases1Medical Biophysics, University of Toronto, Toronto, ON, Canada, 2Radiation Oncology, Sunnybrook Health Sciences Centre, Toronto, ON, Canada, 3Physical Sciences, Sunnybrook Research Institute, Toronto, ON, Canada, 4GE Healthcare, Toronto, ON, Canada, 5Pharmacy, Sunnybrook Health Sciences Centre, Toronto, ON, Canada, 6Radiology, Sunnybrook Health Sciences Centre, Toronto, ON, Canada
Synopsis
Brain metastases are increasingly being treated with stereotactic radiosurgery; however, 20-30% of treated tumors recur locally post-treatment. Hyperpolarized [1-13C]pyruvate magnetic resonance imaging (HP 13C MRI) is an emerging metabolic imaging modality that measures key metabolic phenotypes indicative of tumor biology. Here we investigate pre-treatment [1-13C]pyruvate uptake – a potential marker of monocarboxylate transporter 1 expression and tumor vascularity – via HP 13C MR images as a predictor of local recurrence. [1-13C]pyruvate uptake establishes a robust predictive model (AUC = 0.73) and, as a result, can inform treatment decisions should the model predict a non-response to SRS.
Introduction
Brain metastases (BMs) occur in 20-40% of all cancer patients1–4 and are commonly treated with surgery, stereotactic radiosurgery (SRS), or a combination of the two.1,5 Although SRS has proven a highly effective treatment, 20-30% of BMs recur locally.6 In such cases, an alternative radiation schedule or surgery may have been more beneficial; however, current methods for response assessment rely on detecting changes in tumor volume on follow-up 1H magnetic resonance imaging (MRI).7 Although promising, volumetric changes take several weeks to months to occur. Functional imaging modalities, on the other hand, have the potential to detect treatment response on a shorter timescale. Hyperpolarized [1-13C]pyruvate MRI (HP 13C MRI) is an emerging metabolic imaging modality that not only allows for a 104-fold increase in signal of key metabolites in vivo,8,9 but also for the interrogation of key metabolic branching points that 18F-flurodeoxyglucose positron emission tomography cannot. Recent literature has focused on pyruvate-to-lactate conversion to investigate the extent of anaerobic respiration in tumors and to potentially be used as a marker for disease aggressiveness or response to therapy.10–12 Additionally, our group has shown that pre-treatment [1-13C]lactate production in patients with BMs is predictive of response to SRS (AUC = 0.77, p < 0.05).13 It has been shown, however, that the lactate-to-pyruvate ratio measured by this method in vivo is rate-limited by monocarboxylate transporter 1 (MCT1) expression in the plasma membrane of cells.14,15 This motivates the work presented here, which explores the predictive power of [1-13C]pyruvate uptake as a potential read out of MCT1 expression and tumor vascularity. MCT1 expression16–19 and vascularity20,21 are both upregulated in cancer, are indicators of a highly aggressive phenotype, and can influence response to therapy. We hypothesize that high tumor [1-13C]pyruvate signal on pre-treatment HP [1-13C]pyruvate MRI can predict non-responders to SRS.Methods
Written informed consent was obtained from n=11 BM patients (m=17 tumors) prior to study participation under a protocol approved by the Sunnybrook Research Ethics Board and Health Canada. A 0.43 mL/kg dose of 250mM [1-13C]pyruvate was prepared in a sterile fluid path and hyperpolarized in a GE SPINLab polarizer. Participants were scanned using a GE MR750 3.0T MRI scanner (GE Healthcare, WI) with their head secured in the support of a standard 8-channel neurovascular receive array (Invivo Inc.). A custom 13C birdcage coil was subsequently docked in place. [1-13C]pyruvate was intravenously injected at 4mL/s, followed by a 25mL saline flush at 5mL/s. A 3D dual-echo echo-planar imaging sequence was used to acquire time resolved volumetric [1-13C]pyruvate images (5s temporal resolution; 1.5cm isotropic spatial resolution; 24x24x36cm3 field of view).22 Following 13C image acquisition, the 8-channel 1H neurovascular array was used to acquire 1H T1-w, gadolinium (Gd) enhanced T1-w, and T2-FLAIR images.13C image reconstruction was completed in MATLAB (MathWorks Inc., MA). Time resolved 13C images were summed to compute the area under the curve (AUC) for [1-13C]pyruvate. Tumors were contoured by a radiation oncologist on T1-w or T2-w images if Gd enhanced T1-w images were not available. To obtain regional pyruvate signal information, BrainParser and the LPBA40 brain atlas were used to parcellate T1-w images of the brain into 56 anatomical regions.23 Finally, [1-13C]pyruvate z-scores were calculated for each tumor and brain region (i=57 and 56, respectively) using:
$$z_i = \frac{x_i - \mu}{\sigma}$$
where zi is the ith region’s z-score, xi is the ith region’s mean pyruvate signal, and $$$\mu$$$ and $$$\sigma$$$ are the mean and standard deviation of i-1 regions. Tumor response was determined according to the RANO-BM criteria.7
Results and Discussion
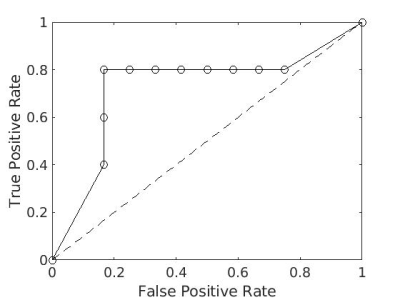
Figure 1 shows the pyruvate z-score pattern across the 56 brain regions for each study participant. This metric is consistent across individuals (Kendall’s W=0.76), which forms the motivation for the use of z-scores. To assess the predictive power of tumor pyruvate, z-scores were computed for each tumor and analyzed using receiver operating characteristics curve (ROC) analysis. Figure 2a shows the z-score for each tumor separated by primary cancer type. The data were scaled between 0 and 1 and pooled together for ROC analysis, as a one-way ANOVA concluded at least one statistically significant difference in pyruvate z-score distributions between primary types (F(3,13)=4.56, p=0.0216) (Figure 2b). Figure 3 shows the resulting ROC curve, with an area under the ROC curve (AUC) of 0.73 and optimal threshold resulting in a true positive rate of 0.8 and false positive rate of 0.17. The statistical significance of this result was evaluated using the Mann-Whitney U Test,24 with $$$\alpha$$$ =0.05. The ROC curve resulted in p=0.08.While [1-13C]pyruvate uptake has classically been used as a normalization signal in the HP 13C MRI literature, for the first time the predictive power of [1-13C]pyruvate uptake normalized to [1-13C]pyruvate in brain parenchyma is explored. Although not as predictive as [1-13C]lactate,13 the higher signal of [1-13C]pyruvate can achieve higher spatial resolution and make imaging lesions smaller than 1cm possible. Future work will investigate the combination of [1-13C]pyruvate and [1-13C]lactate data to improve local recurrence predictions.
Conclusions
The initial experience in using HP [1-13C]pyruvate uptake in brain metastases as a predictive marker for local recurrence is provided. ROC analysis resulted in a robust predictive model, which could potentially inform treatment decisions for tumors that are unlikely to respond to SRS.Acknowledgements
I would like to sincerely thank my supervisor, Dr. Charles Cunningham, for his continued valuable support, ideas, and enthusiasm throughout my graduate studies thus far. My sincere thanks also goes to Dr. Casey Lee, who's PhD thesis laid the ground work for the results presented here. This work would not be possible without Dr. Lee. Thank you to Dr. Hany Soliman for supporting this project and helping with participant recruitment. Finally, thank you to my lab mates, Brin Uthayakumar and Nadia Bragagnolo, who trained me and taught me so much about HP 13C MRI over the past year.References
1. Soliman H, Das S, Larson DA, Sahgal A. Stereotactic radiosurgery (SRS) in the modern management of patients with brain metastases. Oncotarget. 2016;7(11):12318-12330. doi:10.18632/oncotarget.7131
2. Nayak L, Lee EQ, Wen PY. Epidemiology of Brain Metastases. Curr Oncol Rep. 2012;14(1):48-54. doi:10.1007/s11912-011-0203-y
3. Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE. Incidence Proportions of Brain Metastases in Patients Diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. 2004;22(14):2865-2872. doi:10.1200/JCO.2004.12.149
4. Tsukada Y, Fouad A, Pickren JW, Lane WW. Central nervous system metastasis from breast carcinoma autopsy study. Cancer. 1983;52(12):2349-2354. doi:https://doi.org/10.1002/1097-0142(19831215)52:12<2349::AID-CNCR2820521231>3.0.CO;2-B
5. Tsao MN, Rades D, Wirth A, et al. Radiotherapeutic and surgical management for newly diagnosed brain metastasis(es): An American Society for Radiation Oncology evidence-based guideline. Pract Radiat Oncol. 2012;2(3):210-225. doi:10.1016/j.prro.2011.12.004
6. Chao ST, De Salles A, Hayashi M, et al. Stereotactic Radiosurgery in the Management of Limited (1-4) Brain Metasteses: Systematic Review and International Stereotactic Radiosurgery Society Practice Guideline. Neurosurgery. 2018;83(3):345-353. doi:10.1093/neuros/nyx522
7. Lin NU, Lee EQ, Aoyama H, et al. Response assessment criteria for brain metastases: proposal from the RANO group. Lancet Oncol. 2015;16(6):e270-e278. doi:10.1016/S1470-2045(15)70057-4
8. Wolber J, Ellner F, Fridlund B, et al. Generating highly polarized nuclear spins in solution using dynamic nuclear polarization. Nucl Instrum Methods Phys Res Sect Accel Spectrometers Detect Assoc Equip. 2004;526(1-2):173-181. doi:10.1016/j.nima.2004.03.171
9. Ardenkjaer-Larsen JH, Fridlund B, Gram A, et al. Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR. Proc Natl Acad Sci. 2003;100(18):10158-10163. doi:10.1073/pnas.1733835100
10. Granlund KL, Tee SS, Vargas HA, et al. Hyperpolarized MRI of Human Prostate Cancer Reveals Increased Lactate with Tumor Grade Driven by Monocarboxylate Transporter 1. Cell Metab. 2020;31(1):105-114.e3. doi:10.1016/j.cmet.2019.08.024
11. Albers MJ, Bok R, Chen AP, et al. Hyperpolarized 13 C Lactate, Pyruvate, and Alanine: Noninvasive Biomarkers for Prostate Cancer Detection and Grading. Cancer Res. 2008;68(20):8607-8615. doi:10.1158/0008-5472.CAN-08-0749
12. Nelson SJ, Kurhanewicz J, Vigneron DB, et al. Metabolic Imaging of Patients with Prostate Cancer Using Hyperpolarized [1-13C]Pyruvate. Sci Transl Med. 2013;5(198):198ra108-198ra108. doi:10.1126/scitranslmed.3006070
13. Lee CY, Soliman H, Bragagnolo ND, et al. Predicting response to radiotherapy of intracranial metastases with hyperpolarized [Formula: see text]C MRI. J Neurooncol. Published online March 19, 2021. doi:10.1007/s11060-021-03725-7
14. Rao Y, Gammon S, Zacharias NM, et al. Hyperpolarized [1- 13 C]pyruvate-to-[1- 13 C]lactate conversion is rate-limited by monocarboxylate transporter-1 in the plasma membrane. Proc Natl Acad Sci. 2020;117(36):22378-22389. doi:10.1073/pnas.2003537117
15. Harris T, Eliyahu G, Frydman L, Degani H. Kinetics of Hyperpolarized 13C₁-Pyruvate Transport and Metabolism in Living Human Breast Cancer Cells. Proc Natl Acad Sci U S A. 2009;106(43):18131-18136.
16. Chen AP, Chu W, Gu YP, Cunnhingham CH. Probing Early Tumor Response to Radiation Therapy Using Hyperpolarized [1-13C]pyruvate in MDA-MB-231 Xenografts. Monleon D, ed. PLoS ONE. 2013;8(2):e56551. doi:10.1371/journal.pone.0056551
17. Hong CS, Graham NA, Gu W, et al. MCT1 Modulates Cancer Cell Pyruvate Export and Growth of Tumors that Co-express MCT1 and MCT4. Cell Rep. 2016;14(7):1590-1601. doi:10.1016/j.celrep.2016.01.057
18. Ambrosetti D, Dufies M, Dadone B, et al. The two glycolytic markers GLUT1 and MCT1 correlate with tumor grade and survival in clear-cell renal cell carcinoma. Singh PK, ed. PLOS ONE. 2018;13(2):e0193477. doi:10.1371/journal.pone.0193477
19. Romero-Cordoba SL, Rodriguez-Cuevas S, Bautista-Pina V, et al. Loss of function of miR-342-3p results in MCT1 over-expression and contributes to oncogenic metabolic reprogramming in triple negative breast cancer. Sci Rep. 2018;8(1):12252. doi:10.1038/s41598-018-29708-9
20. Song CW, Cho LC, Yuan J, Dusenbery KE, Griffin RJ, Levitt SH. Radiobiology of Stereotactic Body Radiation Therapy/Stereotactic Radiosurgery and the Linear-Quadratic Model. Int J Radiat Oncol. 2013;87(1):18-19. doi:10.1016/j.ijrobp.2013.03.013
21. Park HJ, Griffin RJ, Hui S, Levitt SH, Song CW. Radiation-Induced Vascular Damage in Tumors: Implications of Vascular Damage in Ablative Hypofractionated Radiotherapy (SBRT and SRS). Radiat Res. 2012;177(3):311-327. doi:10.1667/RR2773.1
22. Geraghty BJ, Lau JYC, Chen AP, Cunningham CH. Dual-Echo EPI sequence for integrated distortion correction in 3D time-resolved hyperpolarized 13C MRI. Magn Reson Med. 2018;79(2):643-653. doi:10.1002/mrm.26698
23. Shattuck DW, Mirza M, Adisetiyo V, et al. Construction of a 3D probabilistic atlas of human cortical structures. NeuroImage. 2008;39(3):1064-1080. doi:10.1016/j.neuroimage.2007.09.031
24. Grunkemeier GL, Jin R. Receiver operating characteristic curve analysis of clinical risk models. Ann Thorac Surg. 2001;72(2):323-326. doi:10.1016/S0003-4975(01)02870-3
Figures
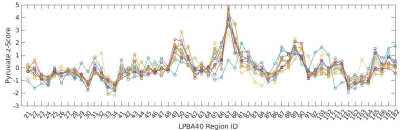
Figure 2: (a) Scaled tumor [1-13C]pyruvate z-scores as a function of primary cancer type. A total of m=17 tumors are included. Non-responding tumors are identified in red. (b) One-way ANOVA results conclude that brain metastases from different primary cancer sites exhibit different [1-13C]pyruvate z-scores.