0570
A 320 km Hyperpolarization Journey: performing [U-13C, d7]glucose DNP in Copenhagen and Hyperpolarized 13C-MR in Aarhus1Physics, EPFL, Lausanne, Switzerland, 2Technical University of Denmark, Kgs. Lyngby, Denmark, 3Clinical Medicine, Aarhus University Hospital, Aarhus, Denmark
Synopsis
Dissolution DNP polarizers are expensive, technically demanding and require trained personnel. At the same time, hyperpolarized MR tracers have a half-life short enough to need the polarizer to be placed as close as possible to the MRI scanner. Similarly to PET tracers, being able to receive on demand hyperpolarized contrast agents from a centralized facility would solve this major shortcoming and boost the diffusion of hyperpolarized MR on a wider scale. In this work, using UV-induced non-persistent radicals and purpose build hardware, we demonstrate the first “across-cities-dDNP” experiment.
Introduction
13C Hyperpolarized Magnetic Resonance (HP-MR) via dissolution Dynamic Nuclear Polarization (dDNP) has the potential of revolutionizing diagnostic radiology.1–3 However, the type of applications and the diffusion of this technique on a wider scale is limited by the short life-time of the HP state after dissolution. Therefore, HP samples have to be prepared as close as possible to the MR apparatus employing an expensive, technically demanding machine with high running costs (i.e. the dDNP polarizer). The latter makes unlikely to equip each MR facility with hyperpolarization. The culprits are the unpaired electron spins present in the DNP sample in form of organic radicals. They are needed for the DNP process to happen inside the polarizer, but they also prevent extraction of the HP sample in the solid state for the sake of storing, transporting and eventually dissolving it far away from the production site. From some years we are working to make hyperpolarization transportable. The idea behind builds on the use of photo-induced thermally-labile free radicals.4–6 As the paramagnetic molecules decompose (quench) at around 190K it is possible to remove them already in the solid state inside the polarizer while retaining the DNP-induced hyperpolarization and increasing the half-life of the sample to tens of hours.7,8 In this work, thanks to purpose built hardware and methodology, we demonstrate the first "across-cities-dDNP" experiment: we produced HP [U-13C, d7] glucose in Copenhagen and performed a HP MR experiment at Aarhus hospital, after a 320 km drive.Methods
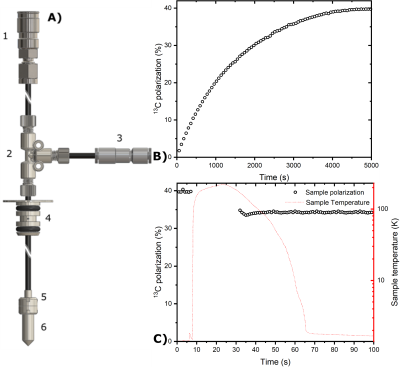
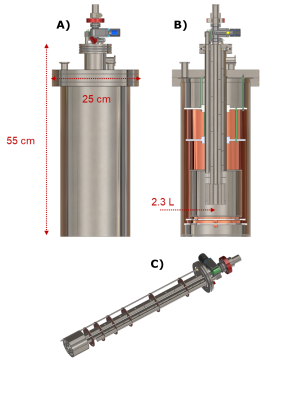
120±5µL of sample containing 2M [U-13C,d7]-D-glucose dissolved in glycerol:water 1:1 (v/v) was polarized via DNP at 1.20±0.05 K and 6.7 T as described earlier.8 The only difference was the employment, as UV-radical precursor, of off the shelf (Sigma Aldrich) trimethylpyruvic acid (TMP) instead of its in-house-synthesized deuterated counterpart. All steps of the experiment (sample loading, polarization, radical scavenging and sample extraction) were accomplished using a reusable custom fluid path (CFP, Figure 1A), equipped with a CERNOX (Lakeshore) sensor to monitor the temperature during the different step of the experiment.8 To extend and monitor the relaxation of the radical free HP sample after extraction, we developed a compact and transportable liquid He bath cryostat equipped with a 1 T Halbach magnet homogeneous enough to perform NMR (Figure 2). The temperature drift of the permanent magnet was characterized using a cryogenic Hall probe (Lakeshore) in combination with 1H-NMR (Kea, Magritek) . After transport (4h), the cryostat was rolled inside the control room of a 3T clinical scanner (GE Healthcare), the dissolution performed using 6mL of 40mM phosphate buffer heated to 170°C, and the HP solution recovered in a 10 mL syringe. The latter was inserted into the scanner bore and the HP 13C signal decay acquired with 20° pulses every 3 s, using a surface coil (Rapid Biomedical).Results and Discussion
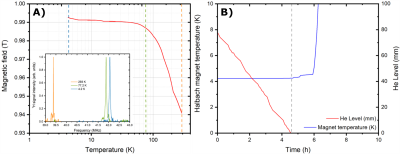
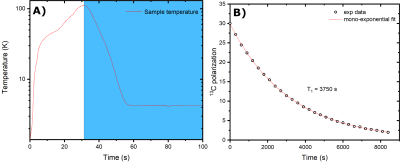
If pre-cooled to 77 K using liquid nitrogen, the cryostat needs 5 L of liquid He to be filled completely. The boil-off rate of 0.3 L/h guarantees to stay at 4.2 K for 7.5 h when the 2.3 L pot is full (Figure 3B). The Halbach magnet drifted from 0.940 T at 293 K to 0.992 T at 4.2 K (Figure 3A). Monitoring the drift allowed us to find a 13C frequency of 10.56 MHz at transport/storage conditions. The sample reached a solid-state polarization of 38±2% with a build-up time constant of 1700±100 s (Figure 1B). Scavenging the radicals by heating the sample above 200 K for 10 s caused a relative polarization loss of 15% (Figure 1C). As earlier demonstrated,8 despite a temperature increase up to 110 K (Figure 4A), extracting the sample after radicals scavenging is essentially lossless, as far as a magnetic field higher than 40 mT is provided. Therefore, as soon as the sample reaches the Halbach magnet inside the cryostat, we estimate a residual 13C polarization of approx. 30%. In disagreement with our former results, the 13C glucose T1 at 1 T and 4.2 K was only 1 h (Figure 4B) compared to the 4h measured inside the polarizer using a field cycling procedure.8 We ascribe this discrepancy to the employment of the protonated version of TMP. Indeed, methyl-groups rotation can represent an additional relaxation mechanism. With this relaxation time value, the residual polarization at the moment of dissolution after 4 h transport is estimated around 0.55%. Inside the clinical scanner, after 10 s transfer time, we measured a glucose 13C polarization of 0.28%. The result is coherent with our estimation, taking into account a glucose liquid-state T1 of 20.3 s (Figure 5).Conclusions and Perspectives
Combining the unique thermal instability of UV-induced radicals to purpose-built hardware, we demonstrated, for the first time, the feasibility of an “across-cities-dDNP” experiment. The final polarization is low compared to HP MR applications standards. The latter was due to a long transport time compared to the T1 value. We plan to repeat the experiment using the deuterated version of the precursor, as soon as the synthesis of a new batch will be ready. Moreover, we plan to attempt the transport of other HP molecules such as pyruvate.Acknowledgements
This work was supported by the Swiss National Science Foundation (under the SPARK grant agreement no. CRSK-2_190547 and Ambizione grant agreement no. PZ00P2_193276, assigned to Capozzi) and The Danish National Research Foundation (DNRF124, assigned to Ardenkjær-Larsen).References
(1) Ardenkjaer-Larsen, J. H.; Fridlund, B.; Gram, A.; Hansson, G.; Hansson, L.; Lerche, M. H.; Servin, R.; Thaning, M.; Golman, K. Increase in Signal-to-Noise Ratio of > 10,000 Times in Liquid-State NMR. Proceedings of the National Academy of Sciences of the United States of America Sep 2, 100 (18), 10158–10163.
(2) Kurhanewicz, J.; Vigneron, D. B.; Ardenkjaer-Larsen, J. H.; Bankson, J. A.; Brindle, K.; Cunningham, C. H.; Gallagher, F. A.; Keshari, K. R.; Kjaer, A.; Laustsen, C.; Mankoff, D. A.; Merritt, M. E.; Nelson, S. J.; Pauly, J. M.; Lee, P.; Ronen, S.; Tyler, D. J.; Rajan, S. S.; Spielman, D. M.; Wald, L.; Zhang, X.; Malloy, C. R.; Rizi, R. Hyperpolarized (13)C MRI: Path to Clinical Translation in Oncology. Neoplasia 2019, 21 (1), 1–16. https://doi.org/10.1016/j.neo.2018.09.006.
(3) Nelson, S. J.; Kurhanewicz, J.; Vigneron, D. B.; Larson, P. E. Z.; Harzstark, A. L.; Ferrone, M.; van Criekinge, M.; Chang, J. W.; Bok, R.; Park, I.; Reed, G.; Carvajal, L.; Small, E. J.; Munster, P.; Weinberg, V. K.; Ardenkjaer-Larsen, J. H.; Chen, A. P.; Hurd, R. E.; Odegardstuen, L. I.; Robb, F. J.; Tropp, J.; Murray, J. A. Metabolic Imaging of Patients with Prostate Cancer Using Hyperpolarized [1-13$C]Pyruvate. Science Translational Medicine Aug 14, 5 (198), 198ra108 1-10. https://doi.org/ARTN 198ra108 DOI 10.1126/scitranslmed.3006070.
(4) Eichhorn, T. R.; Takado, Y.; Salameh, N.; Capozzi, A.; Cheng, T.; Hyacinthe, J. N.; Mishkovsky, M.; Roussel, C.; Comment, A. Hyperpolarization without Persistent Radicals for in Vivo Real-Time Metabolic Imaging. Proceedings of the National Academy of Sciences of the United States of America Nov 5, 110 (45), 18064–18069. https://doi.org/DOI 10.1073/pnas.1314928110.
(5) Capozzi, A.; Karlsson, M.; Petersen, J. R.; Lerche, M. H.; Ardenkjaer-Larsen, J. H. Liquid-State 13 C Polarization of 30% through Photoinduced Nonpersistent Radicals. J. Phys. Chem. C 2018, 122 (13), 7432–7443. https://doi.org/10.1021/acs.jpcc.8b01482.
(6) Capozzi, A.; Patel, S.; Gunnarsson, C. P.; Marco-Rius, I.; Comment, A.; Karlsson, M.; Lerche, M. H.; Ouari, O.; Ardenkjaer-Larsen, J. H. Efficient Hyperpolarization of U-(13) C-Glucose Using Narrow-Line UV-Generated Labile Free Radicals. Angewandte Chemie 2019, 58 (5), 1334–1339. https://doi.org/10.1002/anie.201810522.
(7) Capozzi, A.; Cheng, T.; Boero, G.; Roussel, C.; Comment, A. Thermal Annihilation of Photo-Induced Radicals Following Dynamic Nuclear Polarization to Produce Transportable Frozen Hyperpolarized 13C-Substrates. Nat Commun 2017, 8 (1), 15757. https://doi.org/10.1038/ncomms15757. (8) Capozzi, A.; Kilund, J.; Karlsson, M.; Patel, S.; Pinon, A. C.; Vibert, F.; Ouari, O.; Lerche, M. H.; Ardenkjær-Larsen, J. H. Metabolic Contrast Agents Produced from Transported Solid 13C-Glucose Hyperpolarized via Dynamic Nuclear Polarization. Commun Chem 2021, 4 (1), 95. https://doi.org/10.1038/s42004-021-00536-9.
Figures