0568
On the Feasibility of Hybrid-State based Quantitative MRI at 0.55T1New York University School of Medicine, Center for Biomedical Imaging, New York, NY, United States, 2Center for Advanced Imaging Innovation and Research, New York University School of Medicine, New York, NY, United States
Synopsis
Low field magnetic resonance systems have potential as powerful diagnostic tools at comparably low cost. However, the inherently lower signal requires the use of SNR-efficient pulse sequences and reconstruction schemes. In this work, we present initial results with a hybrid-state free precession sequence, used to quantify T1 and T2 in 12min with an isotropic resolution of 1mm3. Visually, these quantitative maps show low noise levels, which allows for compelling synthetic MP-RAGE contrast generation.
Introduction
Low-field MRI systems have potential as powerful diagnostic tools at lower costs compared to typical MRI systems. However, advanced hardware and software are needed to meet the high clinical standards of image quality and scan time despite the inherently lower signal. In this work, we present the use of highly SNR-efficient quantitative transient-state sequences to mitigate some of the challenges of low-field MRI systems.Theory
Fully balanced SSFP (bSSFP) sequences are well known to be highly SNR-efficient and this sequence design has been used in transient-state quantitative MRI, for example, IR-bSSFP1, MRF2, and hybrid-state free precession (HSFP)3. All of these techniques derive quantitative T$$$_1$$$ and T$$$_2$$$ maps from a single comprehensive scan. This multi-parametric design along with optimized sequence parameters can yield higher SNR in the quantitative maps compared to steady-state approaches3.Decreasing the main magnetic field strength leads to a shorting of T$$$_1$$$ which entails a signal increase in HSFP sequences. The combination of a fully balanced sequence design with the use of a single comprehensive multi-parametric scan could allow HSFP-based approaches to perform well in low-field MRI.
Methods
An HSFP sequence with a radial 3D-kooshball trajectory was used to quantify T$$$_1$$$ and T$$$_2$$$ on a 0.55T prototype MAGNETOM Aera (Siemens Healthcare, Erlangen, Germany), with a nominal resolution of 1mm$$$^3$$$ and an overall scan time of 12min 4s. A flip angle (FA) pattern optimized for the quantification of T$$$_1$$$ and T$$$_2$$$ at 3T3 was reused. This 0.55T protocol was compared to an HSFP experiment at 3T with a protocol optimized for this field strength. At 3T, the nominal resolution was 0.8mm$$$^3$$$, measured in 6min with a TR of 3.9ms and a pulse duration of 0.5ms. The 3T protocol was optimized for k-space coverage due to the high resolution and the short scan time. At 0.5T, we used pulses with 1ms duration due to the system's lower RF-power and a TR of 4.5ms to reduce the sampling bandwidth, increasing the SNR. Each protocol was measured in a healthy volunteer after obtaining written consent in agreement with our IRB.Coefficient images were reconstructed by solving the low-rank (LR) inverse problem with an additional locally low rank (LLR) penalty4,5. The parameter maps were obtained by dictionary matching. The dictionary was constructed through Bloch-simulation, assuming $$$\Delta$$$B$$$_0=0\,$$$Hz and B$$$_1=1$$$.
Further, we quantitatively compared the Cramer-Rao bound (CRB) between the 3T and the 0.5T HSFP protocols. The CRB was calculated based on a Bloch-model and the relaxation times of white matter (WM) obtained at the respective field strength, and while accounting for the expected SNR levels at the different field strengths.
Last, we visually compared a clinical MP-RAGE image to a synthetic MP-RAGE derived from the HSFP sequence. The clinical MP-RAGE was acquired in 4min$$$\,$$$47s at 0.55T. The equation used to simulate the synthetic contrast of the MP-RAGE sequence is: $$$S=M_0(1-2e^{-\frac{TI}{T1}}+e^{-\frac{TR}{T1}})$$$, with TI$$$\,$$$=$$$\,$$$722$$$\,$$$ms and TR$$$\,$$$=$$$\,$$$1500$$$\,$$$ms, which matches the settings of the clinical protocol.
Results and Discussion
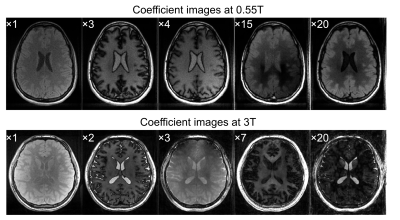
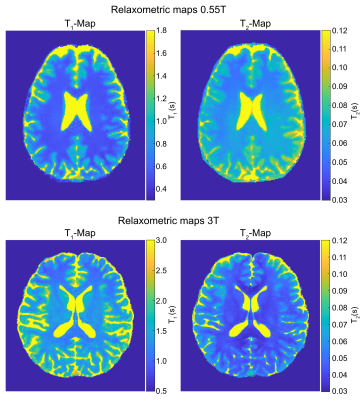
Fig.1 shows coefficient images that were reconstructed from the HSFP sequence. The corresponding quantitative maps are shown in Fig.2 and the mean relaxation times of both protocols are listed in Tab.1. All here presented relaxometry maps are biased by magnetization transfer (MT), although this bias is more pronounced at 3T due to shorter RF-pulses; and the increased MT likely causes the observed bias in T$$$_2$$$ at 3T (Tab.1). Ongoing work of our group models the MT effect, which allows a correction thereof.6Our CRB-based SNR analysis suggests that, given the same resolution and scan time, we achieve a 1.8-fold lower T$$$_1$$$NR and a 4-fold lower T$$$_2$$$NR at 0.55T compared to 3T. While this is a sizable difference, we find that a surprisingly large portion of the $$$B_0$$$-related SNR loss (6-fold as we assume a linear dependency here) is compensated by the faster $$$T_1$$$ relaxation at this field strength. If we account for the difference in resolution and scan time, we actually predict a 1.5-fold higher $$$T_1$$$NR T$$$_1$$$ at 0.55T compared to 3T, while the $$$T_2$$$NR decreases by a factor 0.69. These findings are roughly in line with the experimentally-found values (Tab.1).
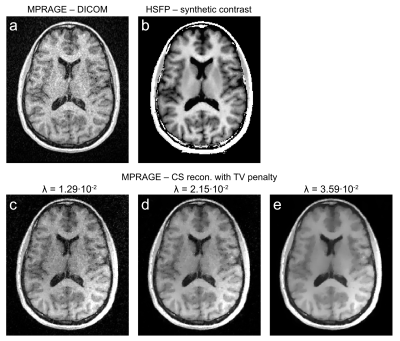
Fig.3 displays the comparison between reconstructions of the clinical MP-RAGE with different amounts of regularization and the synthetic contrast. The synthetic MP-RAGE has higher SNR and delineates subcortical structures better than the clinical scan. Three different regularization parameters are shown to demonstrate that neither results in competitive images. We note, however, that the MP-RAGE has a 2.5-fold shorter scan time. Yet, the HSFP sequence encodes the main contrast mechanisms of multiple clinical sequences, such as FLAIR or T$$$_2$$$-weighted images, which potentially allows the synthesis thereof7,8.
Conclusion
The HSFP sequence allows the acquisition of high-quality T$$$_1$$$ and T$$$_2$$$ maps at 0.55T in 12min with an isotropic resolution of 1mm$$$^3$$$. The CRB analysis revealed that the reduced T$$$_1$$$ at 0.55T compensates for a surprisingly large fraction of the SNR loss due to the lower field strength and our experimental findings are in line with this theoretical prediction. Note that this proof-of-concept work utilized a pulse sequence that was optimized for the relaxation times expected for 3T. Future work will explore further SNR gains by tailoring the RF-pulse train to the relaxation times expected at 0.55T.Acknowledgements
This work was supported by grant NIH/NIBIB R21 EB027241 and was performed under the rubric of the Center for Advanced Imaging Innovation and Research, a NIBIB Biomedical Technology Resource Center (NIH~P41 EB017183).References
1. Ehses P, Seiberlich N, Ma D, et al. IRTrueFISPwith a golden-ratio-based radial readout: Fast quantification of T1, T2, and proton density.Magn. Reson. Med.2013; 69(1): 71–81.
2. Dan Ma, Vikas Gulani, Nicole Seiberlich, Kecheng Liu, Jeffrey L. Sunshine, Jeffrey L. Duerk, and Mark A. Griswold. Magnetic resonance fingerprinting. Nature, 495(7440):187–192, 2013.
3. Jakob Assländer, Dmitry S Novikov, Riccardo Lattanzi, Daniel K Sodickson, and Martijn A Cloos. Hybrid-state free precession in nuclear magnetic resonance. Nat. Commun. Phys., 2(1):73, dec 2019.
4. Jakob Assländer, Martijn A Cloos, Florian Knoll, Daniel K Sodickson, Jürgen Hennig, and Riccardo Lattanzi. Low rank alternating direction method of multipliers reconstruction for MR fingerprinting. Magn. Reson. Med., 79(1):83–96, 2018.
5. Tamir JI, Uecker M, Chen W, et al. T2 shuffling: Sharp, multicontrast, volumetric fast spin-echo imaging.Magn. Reson. Med. 2017.
6. Jakob Assländer and Daniel K Sodickson. Quantitative Magnetization Transfer Imaging in the Hybrid State. In Proc. Intl. Soc. Mag. Reson. Med. 27, page 1104, 2019.
7. K. Wang, M. Doneva, T. Amthor, V. C. Keil, E. Karasan, F. Tan, J. I. Tamir, S. X. Yu, andM. Lustig. High fidelity direct-contrast synthesis from magnetic resonance fingerprinting indiagnostic imaging. InProceedings of the 28th Annual Meeting of ISMRM, volume 867, 2020.
8. G. Wang, E. Gong, S. Banerjee, H. Chen, J. Pauly, and G. Zaharchuk. Oneforall: Improving the quality of multi-contrast synthesized mri using a multi-task generative model. InProceedings ofthe 27th Annual Meeting of ISMRM, 2019.
Figures