0565
Towards quantification of semisolid tissue properties at 7T using multiband-MRF methods.
Daniel West1,2, Raphael Tomi-Tricot3, Lucilio Cordero-Grande1,4,5, Pip Bridgen1, Tom Wilkinson1, Sharon Giles1, Joseph Hajnal1,4, and Shaihan Malik1,4
1Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 2London Collaborative Ultra High Field System (LoCUS), London, United Kingdom, 3MR Research Collaborations, Siemens Healthcare Limited, Frimley, United Kingdom, 4Centre for the Developing Brain, King's College London, London, United Kingdom, 5Biomedical Image Technologies, ETSI Telecomunicación, Universidad Politécnica de Madrid & CIBER-BNN, Madrid, Spain
1Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 2London Collaborative Ultra High Field System (LoCUS), London, United Kingdom, 3MR Research Collaborations, Siemens Healthcare Limited, Frimley, United Kingdom, 4Centre for the Developing Brain, King's College London, London, United Kingdom, 5Biomedical Image Technologies, ETSI Telecomunicación, Universidad Politécnica de Madrid & CIBER-BNN, Madrid, Spain
Synopsis
Since semisolid tissue components are strong determinants of relaxation parameters, estimation of semisolid properties may lead to more robust and direct characterization of tissues than relaxation measurement. We have used multiband MR fingerprinting at ultra-high field in an exploratory study towards high resolution quantitative imaging with efficient parameter encoding. To achieve this, two variants of a steady-state sequence are proposed that employ different cycling of nonselective multiband RF pulses: one to highlight dipolar order effects apparent in lipid bilayer-like materials such as myelin, and the other to measure semisolid T2. Initial phantom results and preliminary in vivo investigation are presented.
Introduction
Semisolid parameter quantification is of importance in MRI since these properties underpin observed relaxation phenomena in tissue1. MR fingerprinting (MRF) sequences are often designed to be maximally sensitive to multiple tissue properties, enabling their simultaneous measurement in an efficient acquisition2. Use of ultra-high field (UHF) strength potentially allows high resolution quantification, however specific absorption rate (SAR) limits are significantly more restrictive compared to clinical field strengths, which imposes constraints on contrasts that rely on RF power deposition, such as inhomogeneous magnetization transfer (ihMT).We propose a combined parameter estimation and motion correction multiband framework at UHF that uses a steady-state sequence with RF pulses comprising a constant on-resonance flip angle but temporally modulated off-resonance bands to create time-variable magnetization-transfer mediated signal modulation3. The signals are sampled using Cartesian encoding and reconstructed with low-rank inversion (LRI)4 and DISORDER motion estimation5. Two variants are explored: the first aims to generate MT and ihMT contrast and is termed MT-MRF-dipolar (similar to Reference 3), whilst the second aims to measure semisolid T2 and is termed MT-MRF-$$$T_2^s$$$.
Methods
This work used a steady-state spoiled gradient echo sequence with either single-band (1B) pulses (on-resonance only) or multiband (2B or 3B) pulses which add one or two off-resonance bands that aim to saturate semisolid magnetization; all pulses are spatially non-selective. MT-MRF-dipolar (Figure 1a) uses short batches of either 3B or 2B pulses interspersed with 1B pulses to allow recovery. 3B pulses have symmetric off-resonance bands, 2B have only either a positive or negative offset - the difference between 3B and 2B gives rise to ihMT contrast6. MT-MRF-$$$T_2^s$$$ uses a similar multiband RF pulse cycling framework but features 2-band pulses for saturation with variable offset frequencies (-10 to 10kHz) to sample the semisolid lineshape at different positions, thus encoding semisolid T2 information (Figure 1b).Phantom and in vivo experiments were performed on a 7T MRI scanner (MAGNETOM Terra, Siemens Healthcare, Erlangen, Germany) with an 8Tx/32Rx channel head coil (Nova Medical, Wilmington, MA, USA). Phantom tubes contained MnCl2-doped water (0.05mM), cross-linked bovine serum albumin (BSA) or prolipid-161 (PL161). The MnCl2 phantom should exhibit no semisolid compartment and BSA should have no dipolar order effects. One healthy volunteer (male aged 32) was scanned according to local ethical approval.
Reconstruction of all data was performed using a combined LRI-DISORDER framework7. The low-rank basis was discovered by using SVD on a coarse dictionary including all model parameters - the number of singular components was chosen to capture ~99% of the energy. Parameter matching used more finely sampled dictionaries with most parameters held fixed and only the following parameters varied: semisolid fraction ($$$M_0^s$$$) and dipolar T1 ($$$T_{1D}^s$$$) for MT-MRF-dipolar, and $$$M_0^s$$$ and semisolid T2 ($$$T_2^s$$$) for MT-MRF-$$$T_2^s$$$. Phantom analysis for the latter used $$$M_0^s$$$ estimates from MT-MRF-dipolar to inform the fit, with frequency-offset of the semisolid line8 also estimated. A B1 map was acquired in vivo using AFI (FA = 60˚) and incorporated during corresponding dictionary matching.
Results
MT-MRF-dipolar phantom results (Figure 2) reveal dipolar contrast in PL161 only and semisolid contrast in both BSA and PL161, as expected. $$$T_{1D}^s$$$ = 24.6±2.0ms in PL161, which concurs with previous measurements at 1.5T3, whilst $$$M_0^s$$$ = 0.15±0.01 in PL161 and $$$M_0^s$$$ = 0.051±0.002 in BSA. $$$T_2^s$$$ maps from MT-MRF-$$$T_2^s$$$ (Figure 2b) show mean values of 8.18±0.39μs in PL161 and 6.03±0.19μs in BSA, with non-negligible semisolid absorption lineshape shift (e.g. -318±10Hz in PL161), though this is likely to depend on B0, which has not yet been accounted for in the dictionary. Both scenarios yield good signal fits despite many remaining model parameters being fixed - normalized residuals are of the order of a few percent.MT-MRF-$$$T_2^s$$$ data can also be processed to yield semiquantitative MT ratio (MTR) maps, calculated as the difference in signal between the ends of the 2-band and 1-band periods. DISORDER reconstruction of an in vivo dataset yields motion traces (Figure 3) and MTR maps (Figure 4). Constrained dictionary fitting provides parameter maps (Figure 5). $$$M_0^s$$$ shows strong gray matter-white matter contrast but $$$T_2^s$$$ contrast is mostly flat.
Discussion
MT-MRF-dipolar and MT-MRF-$$$T_2^s$$$ provide reasonable estimates of some important semisolid tissue properties in phantom experiments. Whilst estimation bias certainly exists given the high dimensionality and thus degeneracy of the assumed tissue model, phantom results agreed with previous findings3 at lower field. In vivo estimation of $$$M_0^s$$$ appears robust though $$$T_2^s$$$ shows little contrast. Quantification of $$$T_2^s$$$ remains an open research question; different studies have reported maps with varying degrees of gray matter-white matter contrast9,10. Future work will involve disentangling the effects of remaining tissue parameters in these MRF methods. T1 has been fixed to literature values at 7T1 to perform the above fitting but can be encoded more effectively using inversion pulses, for instance.Conclusion
This work has demonstrated the feasibility of two novel MRF sequences at 7T both using temporally modulated off-resonance saturation with constant flip angle acquisition. One variant encodes dipolar information and is sensitive to lipid bilayer-like structures, whilst the other has potential for $$$T_2^s$$$ quantification. Future work aims to separate out the effects of other tissue parameters to remove residual estimation biases.Acknowledgements
This work was supported by core funding from the Wellcome/EPSRC Centre for Medical Engineering [WT203148/Z/16/Z], Wellcome Trust Collaboration in Science grant [WT201526/Z/16/Z] and by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London and/or the NIHR Clinical Research Facility. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.References
1. Wang Y, van Gelderen P, de Zwart JA, Duyn JH. B0-field dependence of MRI T1 relaxation in human brain. Neuroimage 2020;213:1–11 doi: 10.1016/j.neuroimage.2020.116700.2. Ma D, Gulani V, Seiberlich N, et al. Magnetic resonance fingerprinting. Nature 2013;495:187–192 doi: 10.1038/nature11971.
3. West DJ, Cruz G, Teixeira RPAG, et al. An MR fingerprinting approach for quantitative inhomogeneous magnetization transfer imaging. Magn. Reson. Med. 2021:1–16 doi: 10.1002/mrm.28984.
4. Assländer J, Cloos MA, Knoll F, Sodickson DK, Hennig J, Lattanzi R. Low rank alternating direction method of multipliers reconstruction for MR fingerprinting. Magn. Reson. Med. 2018;79:83–96 doi: 10.1002/mrm.26639.
5. Cordero-Grande L, Ferrazzi G, Teixeira RPAG, O’Muircheartaigh J, Price AN, Hajnal JV. Motion corrected MRI with DISORDER: Distributed and Incoherent Sample Orders for Reconstruction Deblurring using Encoding Redundancy. Magn. Reson. Med. 2020;84:713–726.
6. Malik SJ, Teixeira RPAG, West DJ, Wood TC, Hajnal JV. Steady‐state imaging with inhomogeneous magnetization transfer contrast using multiband radiofrequency pulses. Magn. Reson. Med. 2019;83:935–949 doi: 10.1002/mrm.27984.
7. West DJ, Cordero-Grande L, Teixeira RPAG, Ferrazzi G, Hajnal JV, Malik SJ. Motion corrected magnetization transfer-mediated fingerprinting (MT-MRF) using DISORDER. Proc. ISMRM 2021
8. Jiang X, van Gelderen P, Duyn JH. Spectral characteristics of semisolid protons in human brain white matter at 7 T. Magn. Reson. Med. 2017;78:1950–1958 doi: 10.1002/mrm.26594.
9. Yarnykh VL. Pulsed Z-spectroscopic imaging of cross-relaxation parameters in tissues for human MRI: theory and clinical applications. Magn. Reson. Med. 2002;47:929–939 doi: 10.1002/mrm.10120.
10. Yarnykh VL. Fast macromolecular proton fraction mapping from a single off-resonance magnetization transfer measurement. Magn. Reson. Med. 2012;68:166–178 doi: 10.1002/mrm.23224.
Figures
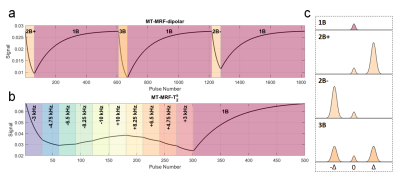
Figure 1: MT-MRF-dipolar and MT-MRF-$$$T_2^s$$$ sequences. For MT-MRF-dipolar (a), RF pulses cycle between 2-band (positive off-resonance, 2B+), 1-band (on-resonance, 1B), 3-band (symmetric off-resonance, 3B) and 2-band pulses (negative off-resonance, 2B-); FA = 5˚, TR = 10ms, pulse duration = 4ms, offset frequency = 7kHz and B1,rms = 1.5μT. MT-MRF-$$$T_2^s$$$ (b) has been optimized for $$$M_0^s$$$ and $$$T_2^s$$$ quantification using CRLB; FA = 7˚, TR = 20ms, pulse duration = 4ms and B1,rms = 1.8/1.6μT for phantom/in vivo acquisitions. Black lines in (a,b) are signal traces.
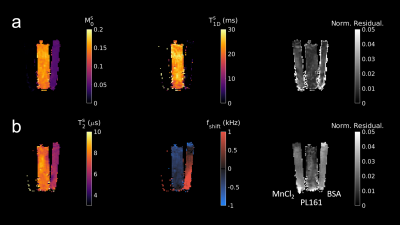
Figure 2: Parameter estimation results from a fit to MT-MRF-dipolar data (a) and MT-MRF-$$$T_2^s$$$ data (b). In each subplot, tubes are in the order specified in the lower right-hand corner.
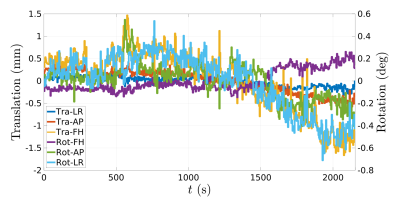
Figure 3: Motion traces from a healthy volunteer, as estimated using the DISORDER reconstruction framework.
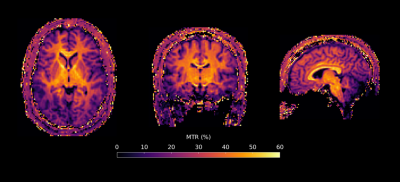
Figure 4: MTR obtained from MT-MRF-$$$T_2^s$$$ in three orientations from a healthy volunteer. Image resolution for this example was 2mm isotropic and total scan time was approximately 30 minutes, though this was a proof-of-concept dataset and we expect that the acquisition can be shortened without image degradation.
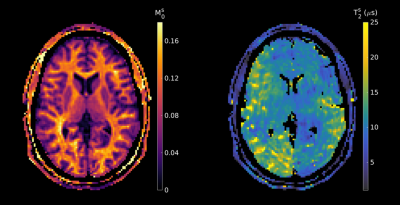
Figure 5: In vivo parameter maps for semisolid fraction and T2. The former shows strong gray-white matter contrast whereas the latter shows lesser variability, potentially indicating the presence of confounding factors in our acquisition. However, $$$T_2^s$$$ estimates of ~9μs in white matter are in-line with values reported in the literature.
DOI: https://doi.org/10.58530/2022/0565