0558
Improving Radiomics Reproducibility Using MR Fingerprinting and Physics-Informed Quantization1Department of Biomedical Engineering, Case Western Reserve University, Cleveland, OH, United States, 2Medical Scientist Training Program, Case Western Reserve University, Cleveland, OH, United States, 3Center for Biomedical Image Computing and Analysis, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, United States, 4Department of Radiology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, United States, 5Siemens Healthineers, Erlangen, Germany, 6Department of Quantitative Health Sciences, Cleveland Clinic, Cleveland, OH, United States, 7Department of Radiology, Case Western Reserve University and University Hospitals Cleveland Medical Center, Cleveland, OH, United States
Synopsis
MR fingerprinting (MRF) is a rapid, quantitative imaging approach with significant potential for use in clinical studies, including radiomic applications. Due to its quantitative nature, robustness to system imperfections, and requiring fewer image preprocessing steps, we believe MRF radiomics is uniquely positioned to offer improved reproducibility and generalizability compared to conventional MRI. Here we report reproducibility results of MRF T1 and T2 radiomic features in the healthy human brain, and introduce a novel physics-informed quantization approach for improved reproducibility of quantitative image texture features.
Introduction
MR fingerprinting (MRF) is a rapid quantitative imaging technique with superior repeatability and reproducibility compared to conventional MRI1–3 and thus significant potential for use in large-scale clinical studies. Previous MRF reproducibility studies have been limited to relaxometry. Because MRF is inherently quantitative, robust to system imperfections4, and does not require significant image preprocessing , we hypothesize that MRF will contribute to more reproducible image analysis and outcome prediction5,6 . This study is a first attempt at comprehensively evaluating the reproducibility of MRF T1 and T2 radiomic features in the healthy human brain. In addition, we propose and evaluate the utility of a novel, physics-informed (PI) quantization approach for more reproducible texture feature calculation of quantitative images.Methods
Study design, imaging protocol, and preprocessingConventional MRI and prototype MRF data from three healthy volunteers were obtained using five distinct 3T MAGNETOM Vida scanners (Siemens Healthcare, Erlangen, Germany). Each volunteer underwent five scans on each scanner, for a total of 25 scans. Examinations included whole-head 3D T1-weighted MPRAGE (0.94x0.94x0.9 mm3) and 2D FISP MRF7,8 (1.0x1.0x5.0 mm3, 13 slices) acquisitions. T1w images were segmented using an atlas-based approach9 and registered to MRF T1 and T2 maps, after which 14 brain regions were identified for analysis.
Quantization approaches for radiomic analysis
Three-dimensional radiomic features were extracted using in-house MATLAB (MathWorks, Natick, MA, USA) software benchmarked to Image Biomarker Standardization Initiative (IBSI) guidelines10. Twenty-five intensity and 74 texture features were extracted for each experimental setting and image type (T1, T2, and T1w).
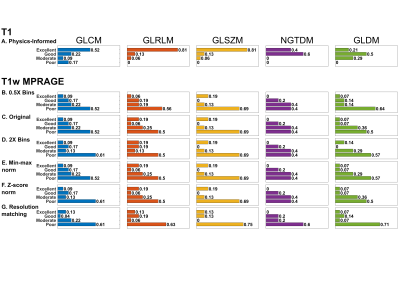
Because of its weighted contrast, T1w texture features were only calculated using conventional fixed bin count quantization: the range of intensities in an ROI are divided into a pre-defined number of equally spaced bins, then intensities are mapped to their corresponding bin (Figure 1). To analyze the effect of preprocessing on texture feature reproducibility from T1w images, radiomic features were also calculated after intensity normalization (min-max or z-score) or after resolution matching to MRF.
The proposed physics-informed (PI) approach (Figure 1) performs quantization using a universally fixed intensity range, bin width, and bin count, and takes advantage of the quantitative nature of MRF: voxel intensities are a direct measure of tissue properties. Thus, an absolute range of MRF measurement values can be identified from a standard reference (for maximum T1 and T2, NMR relaxometry of pure water). A fixed bin width can also be defined using the dictionary step size. Given a fixed range and bin width, a fixed bin count is then defined. Importantly, the intensity range, bin width, and bin count are then universally applied to all images, independent of individual ROI information and without requiring intensity normalization or outlier removal. MRF texture features were calculated using physics-informed (PI) quantization (Figure 1) and fixed bin count quantization.
Statistical analysis
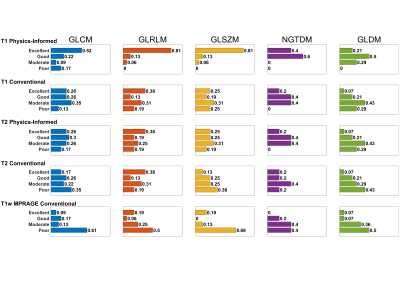
Feature reproducibility was measured via coefficient of variation (CV), the ratio of standard deviation to mean, for T1w images and T1 and T2 maps over each volunteer’s 25 scans. The following CV ranges were defined: excellent (CV < 5%), good (5% <= CV < 10%), moderate (10% <= CV < 20%), and poor (CV >= 20%).
Results
MRF intensity features demonstrate superior reproducibility compared to T1wA comprehensive heatmap of T1, T2, and T1w intensity feature reproducibility is shown in Figure 2. Overall, more T1 (44.9%) and T2 (34.3%) intensity features demonstrated excellent reproducibility compared to T1w (4.9%).
Physics-informed quantization offers tissue-specific physiologic interpretation of and more reproducible MRF radiomic features
Compared to conventional quantization, PI quantization preserves the physiological meaning of quantitative data like between GM and WM regions (Figure 3). PI quantization also allows for greater standardization of radiomic findings and offers reduced sensitivity to sources of study variability such as segmentation errors and measurement outliers (Figure 3).
T1 and T2 texture feature reproducibility in temporal WM following PI and conventional quantization (32 bins) was compared to T1w via thermometer plots with bars representing the proportion of features in each CV range (Figure 4). For all texture families, T1 PI quantization had the greatest proportion of features with excellent reproducibility compared to T1 or T1w conventional quantization.
The effect of varying bin count, intensity normalization, and matching spatial resolution on T1w texture reproducibility was assessed (Figure 5). No appreciable improvement in reproducibility was seen after any operations.
Discussion
This study comprehensively evaluated the reproducibility of MRF radiomics in the healthy human brain: we report superior reproducibility of MRF intensity and texture features compared to conventional MRI. We also introduce a physics-informed (PI) approach to quantitative image quantization that offers several advantages including robustness to outliers and segmentation, reduced sensitivity to preprocessing variation, and preservation of physiological meaning of data. These advantages improve reproducibility of MRF T1 and T2 texture features compared to conventional MRI, even after varying bin count, intensity normalization, and resolution matching.Besides MRF, PI quantization is applicable to any quantitative imaging technique including other quantitative MRI methods or computed tomography (CT). This method thus has considerable application in improving replicability and generalizability of radiomics and machine learning findings.
Conclusion
We report superior reproducibility of MRF radiomic features compared to conventional MRI and demonstrate the significant advantages of our proposed physics-informed radiomics quantization approach.Acknowledgements
This work was supported in part by Siemens Healthineers as well as NIH grants R01 NS109439, R21 EB026764, T32 GM007250, and TL1 TR000441.References
1. Ma D, Gulani V, Seiberlich N, et al. Magnetic resonance fingerprinting. Nature. 2013;495(7440):187-192. doi:10.1038/nature11971
2. Körzdörfer G, Kirsch R, Liu K, et al. Reproducibility and Repeatability of MR Fingerprinting Relaxometry in the Human Brain. Radiology. 2019;292(2):429-437. doi:10.1148/radiol.2019182360
3. Buonincontri G, Biagi L, Retico A, et al. Multi-site repeatability and reproducibility of MR fingerprinting of the healthy brain at 1.5 and 3.0 T. NeuroImage. 2019;195:362-372. doi:10.1016/j.neuroimage.2019.03.047
4. Buonincontri G, Sawiak SJ. MR fingerprinting with simultaneous B1 estimation. Magn Reson Med. 2016;76(4):1127-1135. doi:10.1002/mrm.26009
5. Park JE, Kickingereder P, Kim HS. Radiomics and Deep Learning from Research to Clinical Workflow: Neuro-Oncologic Imaging. Korean J Radiol. 2020;21(10):1126-1137. doi:10.3348/kjr.2019.0847
6. Hoebel KV, Patel JB, Beers AL, et al. Radiomics Repeatability Pitfalls in a Scan-Rescan MRI Study of Glioblastoma. Radiol Artif Intell. 2020;3(1):e190199. doi:10.1148/ryai.2020190199
7. Jiang Y, Ma D, Seiberlich N, Gulani V, Griswold MA. MR fingerprinting using fast imaging with steady state precession (FISP) with spiral readout. Magn Reson Med. 2015;74(6):1621-1631. doi:10.1002/mrm.25559
8. Yokota Y, Okada T, Fushimi Y, et al. Acceleration of 2D-MR fingerprinting by reducing the number of echoes with increased in-plane resolution: a volunteer study. Magma N Y N. 2020;33(6):783-791. doi:10.1007/s10334-020-00842-8
9. Schmitter D, Roche A, Maréchal B, et al. An evaluation of volume-based morphometry for prediction of mild cognitive impairment and Alzheimer’s disease. NeuroImage Clin. 2014;7:7-17. doi:10.1016/j.nicl.2014.11.001
10. Zwanenburg A, Vallières M, Abdalah MA, et al. The Image Biomarker Standardization Initiative: Standardized Quantitative Radiomics for High-Throughput Image-based Phenotyping. Radiology. 2020;295(2):328-338. doi:10.1148/radiol.2020191145
Figures
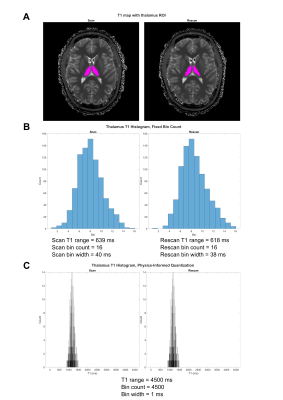
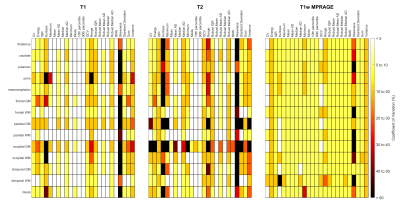
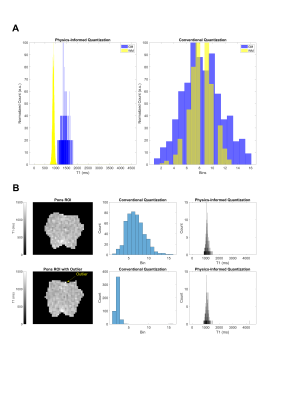
Figure 3. Benefits of physics-informed (PI) quantization for texture feature calculation. (A) PI quantization of T1 GM and WM shows clear separation of the two tissues and reveals key differences in T1 distribution. Conventional quantization leads to loss of these discriminating features. (B) PI quantization is insensitive to segmentation error or outliers, with a trivial change in the T1 intensity distribution. In comparison, there is drastic distortion following conventional quantization.